Question: + A process for methanol synthesis is shown in Figure P12.25. The pertinent chemical reactions involved are CH,2H,0-C, +4# (main reformer reaction) CH+H, 000+ 3H
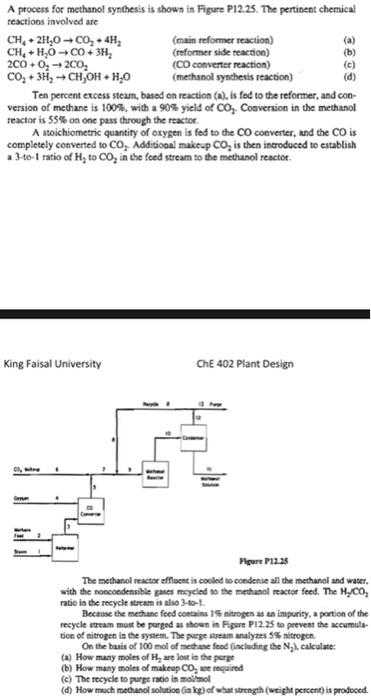
+ A process for methanol synthesis is shown in Figure P12.25. The pertinent chemical reactions involved are CH,2H,0-C, +4# (main reformer reaction) CH+H, 000+ 3H (reformer side reaction) 2000, 200, (CO converter reaction) (c) CO2 + 3H2 - CH,OH + H20 (methanol synthesis reaction) (d) Ten percent excess stean, based on reaction (s), is fed to the reformer, and con- version of methane is 100%, with a 90% yield of Co, Conversion in the methanol reactor is 55% on one pass through the reactor A stoichiometric quantity of oxygen is fed to the CO converter, and the CO is completely converted to CO, Additional makeup Co, is then introduced to establish a 3-to-1 ratio of H, to CO, in the feed stream to the methanol reactor King Faisal University CHE 402 Plant Design mm Figure P12:28 The methanol teactor effluent is cooled to condense all the methanol and water. with the noncondensible gates recycled to the methanol reactor feed. The HyCo, ratio in the recycle stream is also 3-to-1 Because the methanc feed contains 15 nitrogen as an impurity, a portion of the recycle stream must be parged as shown in Figure P12.25 to prevent the accumula- tion of nitrogen in the system. The pegeream analyzes 5 sitrogen On the basis of 100 mol of these feed (including the N.), calculate: (a) How many moles of H, are lost in the perge (b) How many moles of makeup CO, se required (c) The recycle to purge ratio in mol/mol (1) How much mechanol solutice din ) of what strength (weight percent) is produced
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


