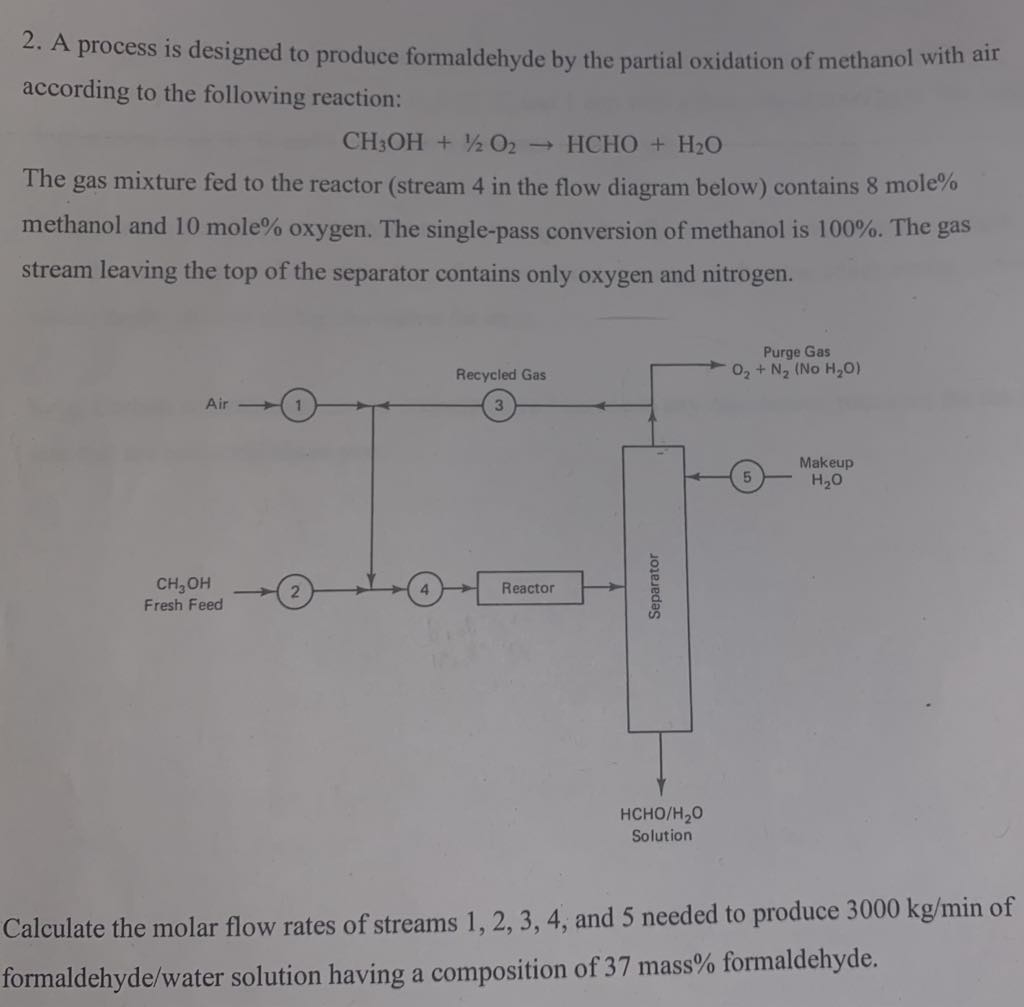
Question: A process is designed to produce formaldehyde by the partial oxidation of methanol with air according to the following reaction: C H 3 O H
A process is designed to produce formaldehyde by the partial oxidation of methanol with air according to the following reaction:
HCHO
The gas mixture fed to the reactor stream in the flow diagram below contains mole methanol and mole oxygen. The singlepass conversion of methanol is The gas stream leaving the top of the separator contains only oxygen and nitrogen.
Calculate the molar flow rates of streams and needed to produce of formaldehydewater solution having a composition of mass formaldehyde.A process is designed to produce formaldehyde by partial oxidation of methanol with air according to the following reaction: CHOH O HCHO H The gas mixture fed to the reactor stream in the flow diagram below contain mole methanol and mole oxygen. The singlepass conversion of methanol is The gas stream leaving the top of the separator contains only oxygen and nitrogen. Calculate the molar flow rates of stream and needed to produce kgmin of formaldehydewater solution having a composition of mass formaldehyde.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


