Question: I want a process flow diagram that describes this process. Formaldehyde is being produced by vapor phase oxidation of methanol according to the following reaction:
I want a process flow diagram that describes this process.
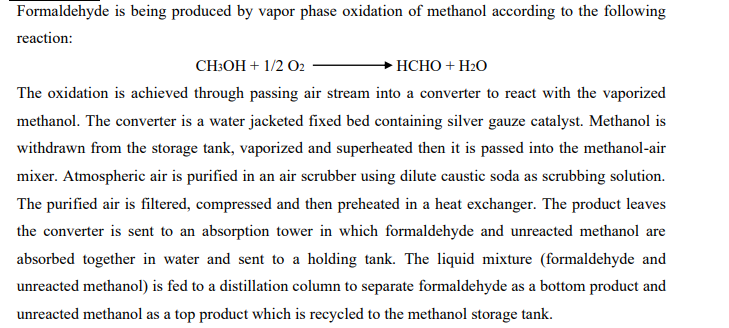
Formaldehyde is being produced by vapor phase oxidation of methanol according to the following reaction: CH3OH + 1/2 02 HCHO + H2O The oxidation is achieved through passing air stream into a converter to react with the vaporized methanol. The converter is a water jacketed fixed bed containing silver gauze catalyst. Methanol is withdrawn from the storage tank, vaporized and superheated then it is passed into the methanol-air mixer. Atmospheric air is purified in an air scrubber using dilute caustic soda as scrubbing solution. The purified air is filtered, compressed and then preheated in a heat exchanger. The product leaves the converter is sent to an absorption tower in which formaldehyde and unreacted methanol are absorbed together in water and sent to a holding tank. The liquid mixture (formaldehyde and unreacted methanol) is fed to a distillation column to separate formaldehyde as a bottom product and unreacted methanol as a top product which is recycled to the methanol storage tank. a
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


