Question: a Question 3 A batch reactor experiment was carried out to determine the specific rate constant, k. It was conducted isothermally at 25 C The
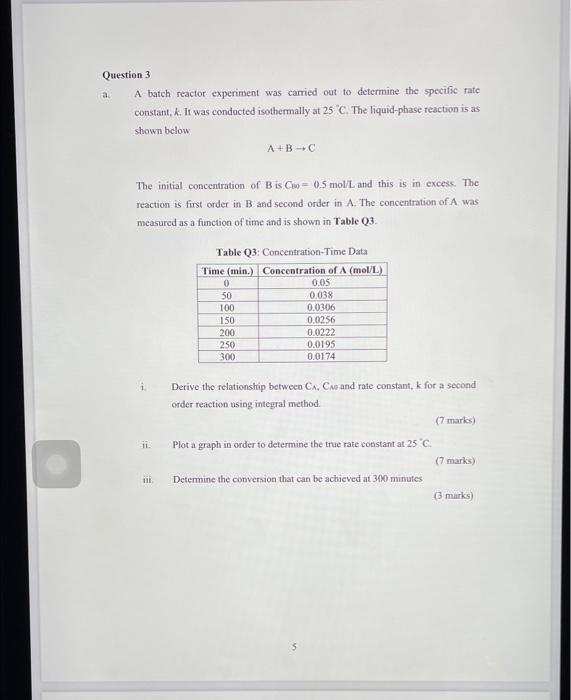
a Question 3 A batch reactor experiment was carried out to determine the specific rate constant, k. It was conducted isothermally at 25 C The liquid-phase reaction is as shown below A+B-C The initial concentration of Bis Cho 0.5 mol/L and this is in excess. The reaction is first order in B and second order in A. The concentration of A was measured as a function of time and is shown in Table 03. Table 03: Concentration Time Data Time (min.) Concentration of A (mol/L) 0 0.05 50 0.038 100 0.0306 150 0.0256 200 0.0222 250 0.0195 300 0.0174 i Derive the relationship between CA, CA and rate constant, k for a second order reaction using integral method. (7 marks) in. Plot a graph in order to determine the true rate constant at 25C. (7 marks) Determine the conversion that can be achieved at 300 minutes 3 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


