Question: Example 5-1. (1) What would be the error in k if the batch reactor were only 80% filled with the same concentrations of reactants, instead
Example 5-1. (1) What would be the error in k if the batch reactor were only 80% filled with the same concentrations of reactants, instead of being completely
filled as in the example?
(2) What generalizations can you draw from this example?
Example 5-1
It is desired to design a CSTR to produce 200 million pounds of ethylene glycol per year by hydrolyzing ethylene oxide. However, before the design can be carried out, it is necessary to perform and analyze a batch-reactor experiment to determine the specific reaction-rate constant, k. Because the reaction will be carried out isothermally, the specific reaction rate will need to be determined only at the reaction temperature of the CSTR. At temperatures above 80°C, there is a significant byproduct formation, while at temperatures below 40°C, the reaction does not proceed at a significant rate; consequently, a temperature of 55°C has been chosen. Because water is present in excess, its concentration (55.5 mol/dm3) may be considered constant during the course of the reaction. The reaction is first-order in ethylene oxide.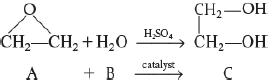
In the laboratory experiment, 500 mL of a 2 M solution (2 kmol/m3) of ethylene oxide
(A) In water was mixed with 500 mL of water
(B) Containing 0.9 wt % sulfuric acid, which is a catalyst. The temperature was maintained at 55°C. The concentration of ethylene glycol
(C) Was recorded as a function of time.
1. Derive an equation for the concentration of ethylene glycol as a function of time.
2. Rearrange the equation derived in (a) to obtain a linear plot of a function concentration versus time.
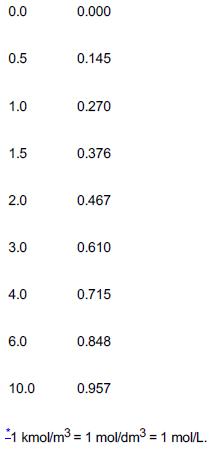
3. Using the data in Table E5-1.1, determine the specific reaction rate, k, at 55°C.
TABLE E5-1.1 CONCENTRATION–TIME DATA
Time (min) Concentration of Ethylene Glycol (C) (kmol/m3)*
Table E5-1.1
Check 10 types of homework problems on the CRE Web site for more solved examples using this algorithm. (http://www.umich.edu/~elements/6e/probsolv/tentypes/index.htm)
CH,CH,+H,O A +B HSO4 catalyst CH-OH CH-OH
Step by Step Solution
3.53 Rating (156 Votes )
There are 3 Steps involved in it
There would be n... View full answer

Get step-by-step solutions from verified subject matter experts


