Question: A sample with an unknown concentration of Cr(NO), has an absorbance of 0.68 at A of 426 nm. A calibration curve was produced for
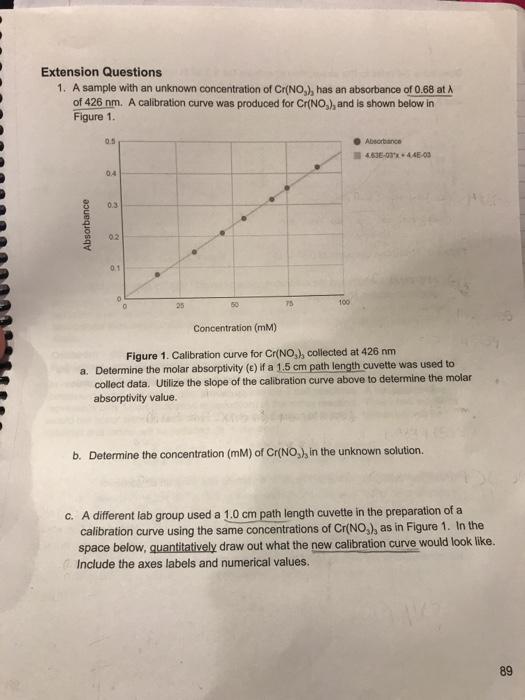
A sample with an unknown concentration of Cr(NO), has an absorbance of 0.68 at A of 426 nm. A calibration curve was produced for Cr(NO), and is shown below in Figure 1. Absorbance 0.5 04 0.3 02 a 01 Absorbance 4636-03x+4.45-03 Concentration (mm) Figure 1. Calibration curve for Cr(NO), collected at 426 nm a. Determine the molar absorptivity (e) if a 1.5 cm path length cuvette was used to collect data. Utilize the slope of the calibration curve above to determine the molar absorptivity value. b. Determine the concentration (mM) of Cr(NO), in the unknown solution. c. A different lab group used a 1.0 cm path length cuvette in the preparation of a calibration curve using the same concentrations of Cr(NO), as in Figure 1. In the space below, quantitatively draw out what the new calibration curve would look like. Include the axes labels and numerical values. 89
Step by Step Solution
3.49 Rating (152 Votes )
There are 3 Steps involved in it
The detailed answer for the above question is provided b... View full answer

Get step-by-step solutions from verified subject matter experts


