Question: A saturated MgSO4 solution at 130F is fed to a crystallizer operating at 50F. The solution leaving the crystallizer is saturated. Magnesium sulfate solubilities are
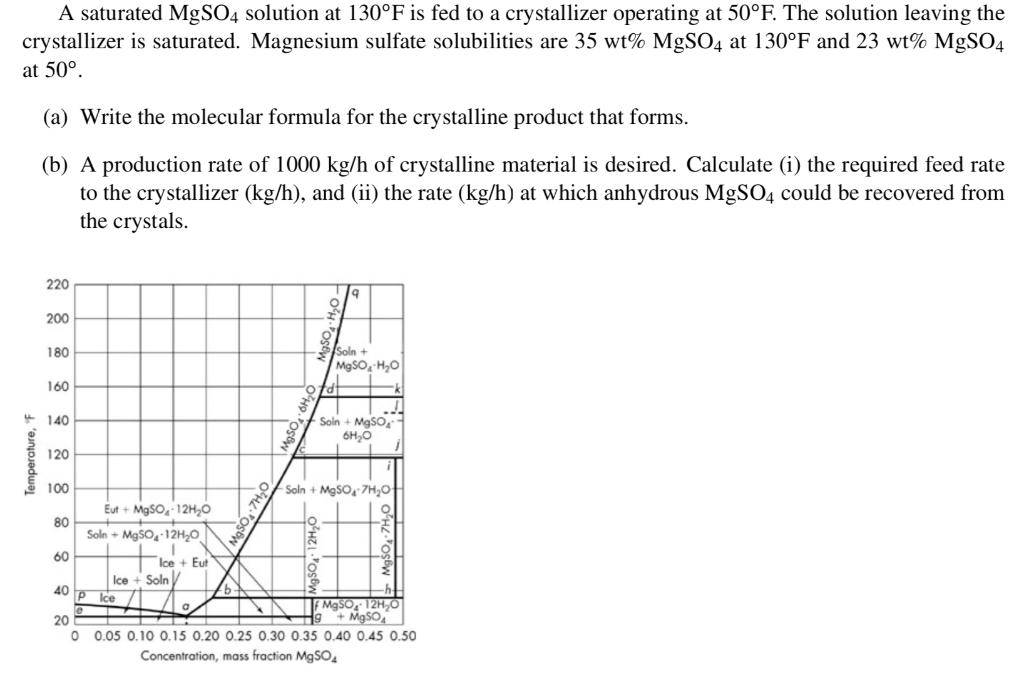
A saturated MgSO4 solution at 130F is fed to a crystallizer operating at 50F. The solution leaving the crystallizer is saturated. Magnesium sulfate solubilities are 35 wt% MgSO4 at 130F and 23 wt% MgSO4 at 50 (a) Write the molecular formula for the crystalline product that forms. (b) A production rate of 1000 kg/h of crystalline material is desired. Calculate (i) the required feed rate to the crystallizer (kg/h), and (ii) the rate (kg/h) at which anhydrous MgSO4 could be recovered from the crystals. 220 9 200 180 Soln MgSO,H,O 160 fd 140 SolnMg50 61,0 Temperature, 120 MgSO, 720 100 Soln + MgSOx7H2O1 Eut + MgSO4-12H,0 80 Soln - Mg5012H2O 60 Ice + Eut Ice + Soln 40 P ide MgSO, 12H 0 20 Hg+ MgSO 0 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40 0.45 0.50 Concentration, mass fraction MgSo
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


