An ore containing 90 wt% MgSO 4 ? 7H 2 O and the balance insoluble minerals is
Question:
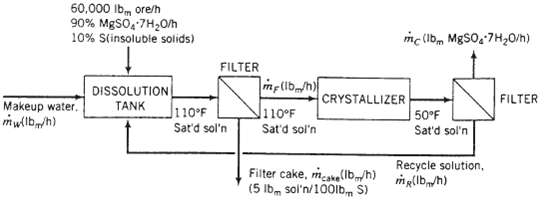
An ore containing 90 wt% MgSO4 ? 7H2O and the balance insoluble minerals is fed to a dissolution tank at a rate of 60,000lbm/h along with fresh water and a recycle stream. The tank contents are heated to 110?F, causing all of the magnesium sulfate heptahydrate in the ore to dissolve, forming a saturated solution. The resulting slurry of the insoluble minerals in saturated MgSO4 solution is pumped to a heated filter, where a wet filter cake is separated from a solids-free filtrate. The filter cake retains 5lbm of saturated solution per 100 lbm of minerals. The filtrate is sent to a crystallizer in which the temperature is reduced o5O?F, producing a slurry of MgSO4?7H2O crystals in a saturated solution that is sent to another filter. The product filter cake contains all of the crystals and entrained solution, again in a ratio of 5 lbm solution per 100lbm crystals. The filtrate from this filter is returned to the dissolution tank as the recycle stream. Solubility data: Saturated magnesium sulfate solutions at 110?F and 50?F contain 32 wt% MgSO4 and 23 wt% MgSO4, respectively.
(a) Explain why the solution is first heated (in the dissolution tank) and filtered and then cooled (in the crystallizer) and filtered.
(b) Calculate the production rate of crystals and the required feed rate of fresh water to the dissolution tank. (Don?t forget to include water of hydration when you write a mass balance on water.)
(c) Calculate the ratio lbm recycle/lbm makeup water.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





