Question: A second - order irreversible liquid - phase reaction A + 2 B - > C with a stoichiometric feed of A and B is
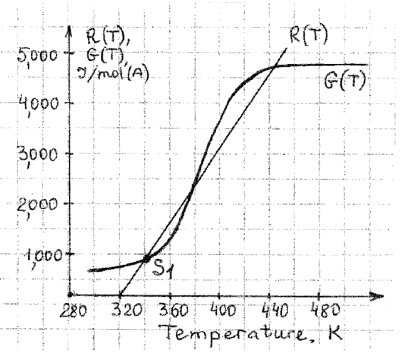
A secondorder irreversible liquidphase reaction ABC with a stoichiometric feed of A and B is carried out in a jacketed CSTR Molar flow rate of A is mols Constant heat capacities are and Jmol K for A B and C respectively. The temperature in the jacket is K Typically the CSTR operated at the steady state S shown below. You can use the graph to plot necessary curves.
Be careful, the graph uses G and R instead of qG and qR These values are normalized according to NAf eg GqGNAf The units are thus slightly different.
a Find the heat of the reaction.
b Find the UA term.
c Assume there was an uncontrolled increase of the feed temperature and the reaction ran away ignited
What should be the temperature of the feed so that the system returns to a desirable locus of conversions?
d Assuming that the reactor operates adiabatically at the feed temperature found in c Write down the equation of the adiabatic operating line with all values entered
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


