Question: A solid metal M can form two oxides, MO and M304. The metal can exist in equilibrium with one of these oxides at different temperatures.
A solid metal M can form two oxides, MO and M304. The metal can exist in equilibrium with one of these oxides at different temperatures. Determine which of the two oxides is in equilibrium with the metal at room temperature and the maximum temperature at which this oxide is in equilibrium with M.
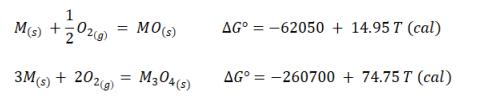
MO(s) AG 3-62050 + 14.95T (l) M(s) +2 M304(3) AG = -260700 + 74.75 T (cal) 3Ms) + 202) %3D
Step by Step Solution
There are 3 Steps involved in it
To determine which oxide is in equilibrium with the metal at room temperature we need to consider th... View full answer

Get step-by-step solutions from verified subject matter experts


