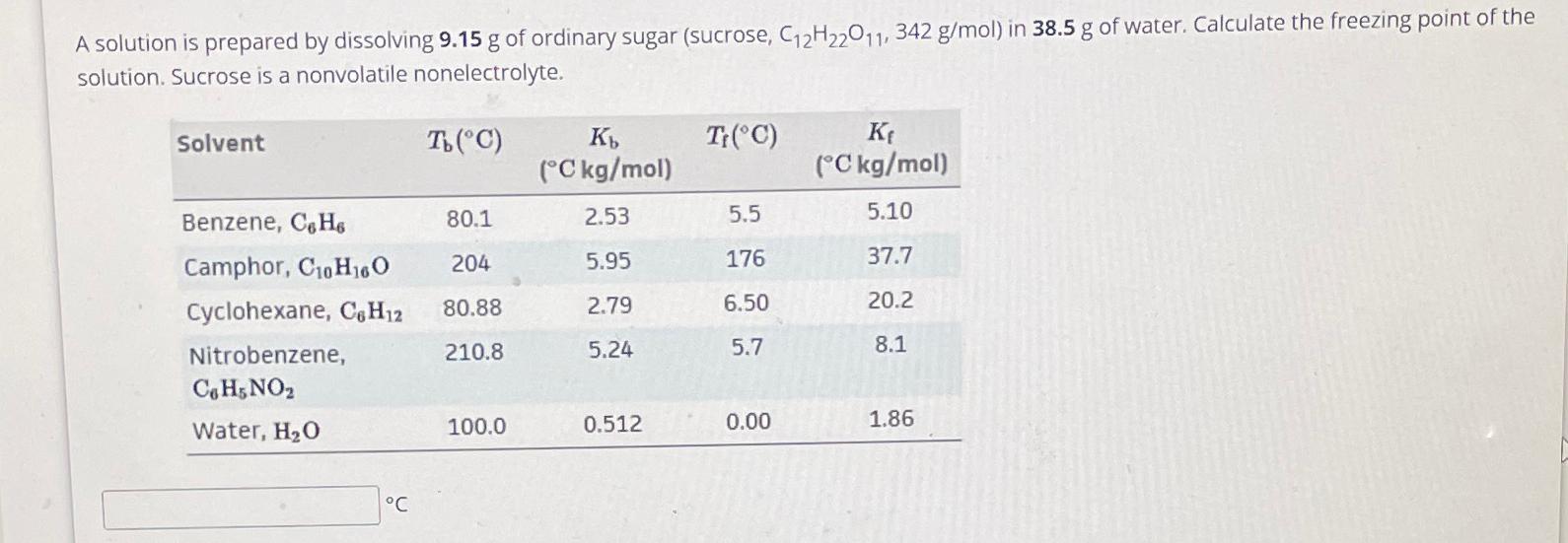
Question: A solution is prepared by dissolving 9 . 1 5 g of ordinary sugar ( sucrose , C 1 2 H 2 2 O 1
A solution is prepared by dissolving of ordinary sugar sucrose in of water. Calculate the freezing point of the solution. Sucrose is a nonvolatile nonelectrolyte.
tableSolventtable
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


