Question: A solution is prepared that is initially 0.47M in acetic acid (HCH3CO2) and 0.43M in sodium acetate (NaCH3CO2). Complete the reaction table below, so that
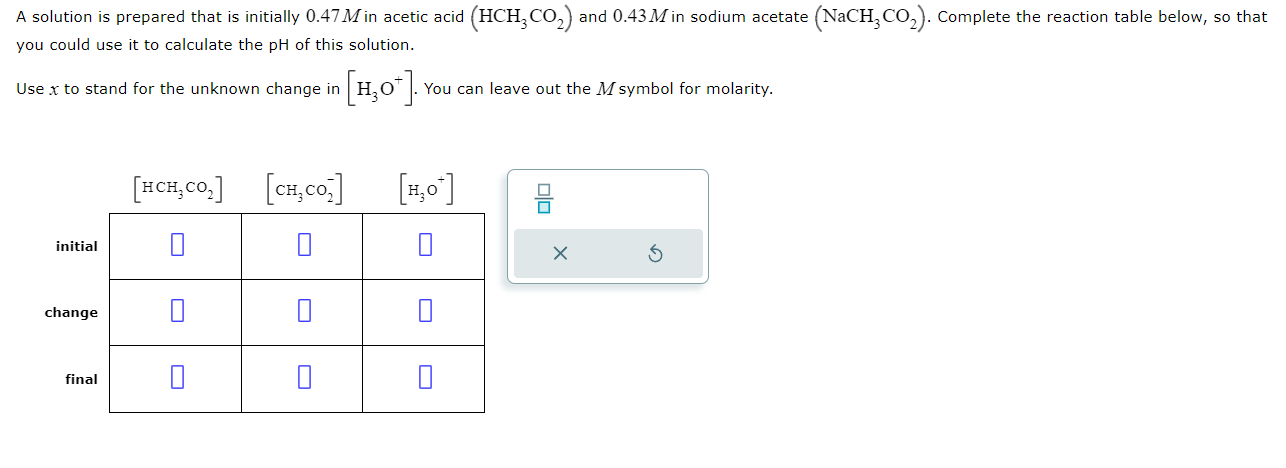
A solution is prepared that is initially 0.47M in acetic acid (HCH3CO2) and 0.43M in sodium acetate (NaCH3CO2). Complete the reaction table below, so that you could use it to calculate the pH of this solution. Use x to stand for the unknown change in [H3O+]. You can leave out the M symbol for molarity
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


