Question: The boiling range is 25 degrees celcius - 33 degrees celcius. The mass of the product was 1.27 grams. Find the calculations for the adjusted
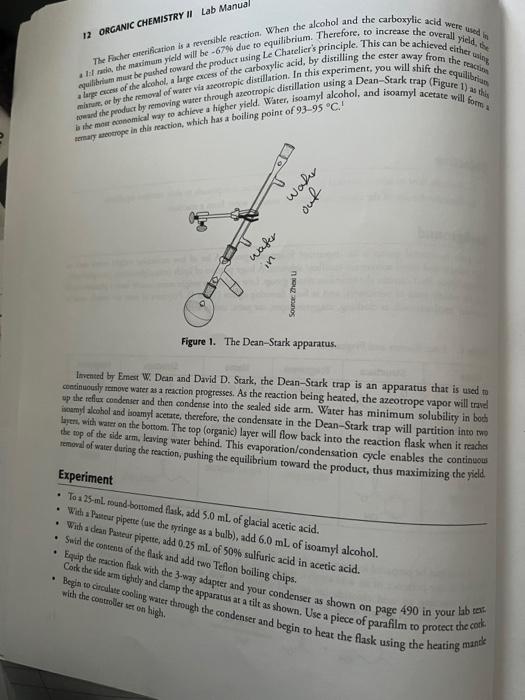

Objective - To illustrare how to utilize physical properties of organic compounds to shift reaction equilibria. - To illuserare the use of the Dean-Stark trap to remove water from a reaction. Background The ester functional group is an important functional group. A lot of low molecular weight esters such as echyl acetate and butyl acetate are excellent solvents. A huge portion of lipids (triacylglycerides, phospholipids, sphingolipids, etc.) contain the ester functional group. Low molecular weight esters usually have a plessant smell and are widely used in perfumery, and as food flavoring agents (Table 1). Polymers of ester are widely used in everydy products such as doching, magnetic tapes, car parts, and so forth. Table 1. Ester Flavors and Fragranoes Eirers can be prepared in a myriad of ways. Two common methods are by reacting a carboxylic aclid with an alcohol in the presence of an acid catalyst (usually H2SO4 ), commonly referred to as the Fischer esterification, and by reacting an acid chloride (or an acid anhydride) with an alcohol. In this experiment, you will be gnthesizing isoamyl acetate using isoamyl alcohol and acetic acid using the Fischer esterification protocol: 12 ORGANIC CHEMISTRY II Lab Manual The Fadher ocerification is a reversible reaction. When the alcohol and the carboxylic acid were uhed in Inrenced by Emest W. Dean and David D. Stark, the Dean-Stark trap is an apparatus that is used to cetinuousty menove water as a reaction progresses. As the reaction being heated, the azeotrope vapor will traid up the refur condenser and then condense into the scaled side arm. Water has minimum solubility in booh besmy a loobal and ioamy acetate, therefore, the condensate in the Dean-Stark trap will partition into two be mop of wate on the bottom. The top (organic) layer will flow back into the reaction flask when it racha renoval of water dutuing the reaction, pushind. This evaporation/condensation cycle enables the continuow the product, thus maximizing the yiald. - Toa 25-mL, round borrotied flask, add 5.0mL of glacial acetic acid. - With a Preser piperte (use the oringe as a balb), add 6.0mL of isoamyl alcohol. - With a dean Partour pipeter, add 0.25mL of 50% sulfuric acid in acetic acid. - Equip the rection fore flask and add two Teflon boiling chips. - Conk the ide am eightly and clamp 3 -way adapeer and your condenser as shown on page 490 in your lab teaf. with the costeroller colling water throwe apparatus at a tilt as shown. Use a piece of parafilm to protect the cotk - After the side arm of the 3-way adapter fills with liquid, continue to heac the reaction for 1h. The distillate is a ternary axeotrope of water-isoamyl alcohol-isoamyl acetate (45:31:24) that boils at 94C. As the distillate collects in the side arm of the adaprer, the lighter organic compounds, isoamyl alcohol and isoamyl acetate, will float on the denser water layer and overflow back inco the flask, The water will be "trapped", therefore, driving the equilibrium. - Let the apparatus cool, and remove the flask. Transfer the contents of the flask to a separatory funnel and wash twice with 5-mL portions of H2O. Drain the H2O layer. Now wash the remaining organic layer twice with 5 -mL portions of sodium bicarbonate solution. [Caution: CO2 will be evolved during the bicarbonate washings] Drain the lower layers each time. - Drain the remaining organic layer into a small Erlenmeyer flask and dry the liquid with 0.5 g of sodium sulfate, Swirl for 12 min. - Transfer the liquid via a Pasteur pipette to a 10 -mL round-bottomed flask and equip the flask for short path distillation (ice-cooled receiver). - Disrill the product into a preweighed 10-mL flask. DO NOT DISTILL. TO DRYNESS. - Record the boiling point range (start to finish). - Obrain the weight of the product. Laboratory Notebook - Use the format provided in the syllabus packet. Make sure you have the boiling points for all materials involved, so you can tuderstand the usefulness of the azcotropic distillation. Product - The observed boiling point for the product. Calculations for theoretical and percent yield, as well as the calculations to determine the limiting reagent. Reference 1. Chiang, S. F.; Chien, L. K; Cheng, C. Y.; Wong, D. D. H. Ind. Eng. Chem. Res, 2002, 41, 3233-3246
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


