Question: A solution of 0.1 M weak base (B) is titrated with 0.1 M of a strong acid (HA). The variation of pH of the
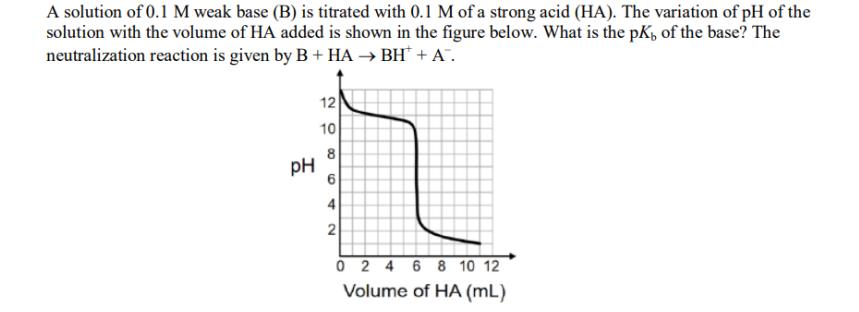
A solution of 0.1 M weak base (B) is titrated with 0.1 M of a strong acid (HA). The variation of pH of the solution with the volume of HA added is shown in the figure below. What is the pK, of the base? The neutralization reaction is given by B + HA BH + A. PH 12 10 8 6 4 2 0 2 4 6 8 10 12 Volume of HA (mL)
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


