Question: A solution was made by adding 11.022g of NaCl into 3.967105g of H2O. Calculate the concentration of NaCl in parts per million, ppm? Question 12
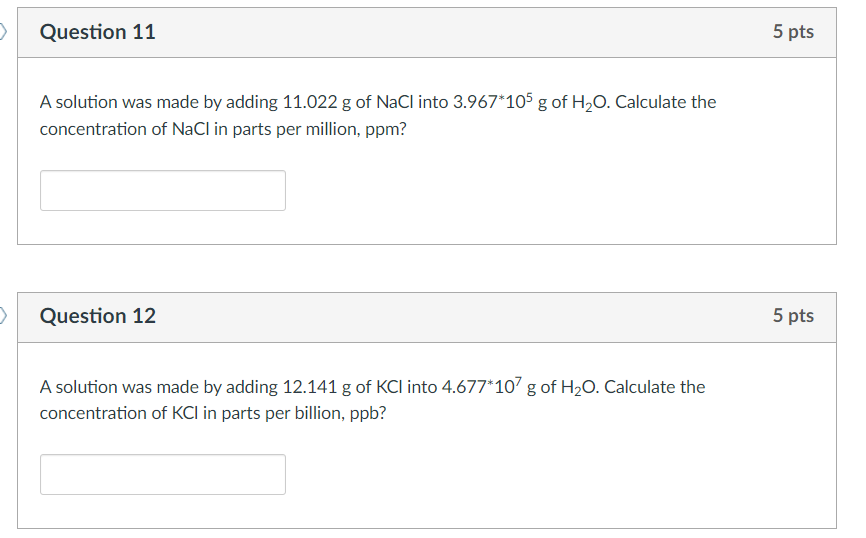
A solution was made by adding 11.022g of NaCl into 3.967105g of H2O. Calculate the concentration of NaCl in parts per million, ppm? Question 12 A solution was made by adding 12.141g of KCl into 4.677107g of H2O. Calculate the concentration of KCl in parts per billion, ppb
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


