Question: A solution was prepared such that the initial concentrations of Cu 2+(aq) and CN aq) were 0.0120 M and 0.0400 M, respectively. These ions react
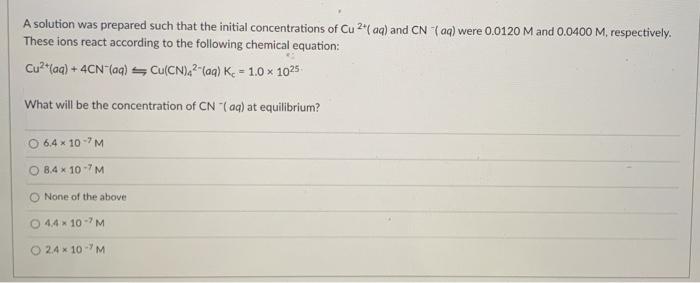
A solution was prepared such that the initial concentrations of Cu 2+(aq) and CN aq) were 0.0120 M and 0.0400 M, respectively. These ions react according to the following chemical equation: Cu?"(aq) + 4CN-(aq) Cu(CN) 2-lag) K - 1.0 1025 What will be the concentration of CN (aq) at equilibrium? 6.4 10-'M 8.4 * 107M None of the above 44 10M 24* 10M
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


