Question: A stream containing 4 0 mole % decane ( C 1 0 H 2 2 ) and 6 0 mole % nonene ( C 9
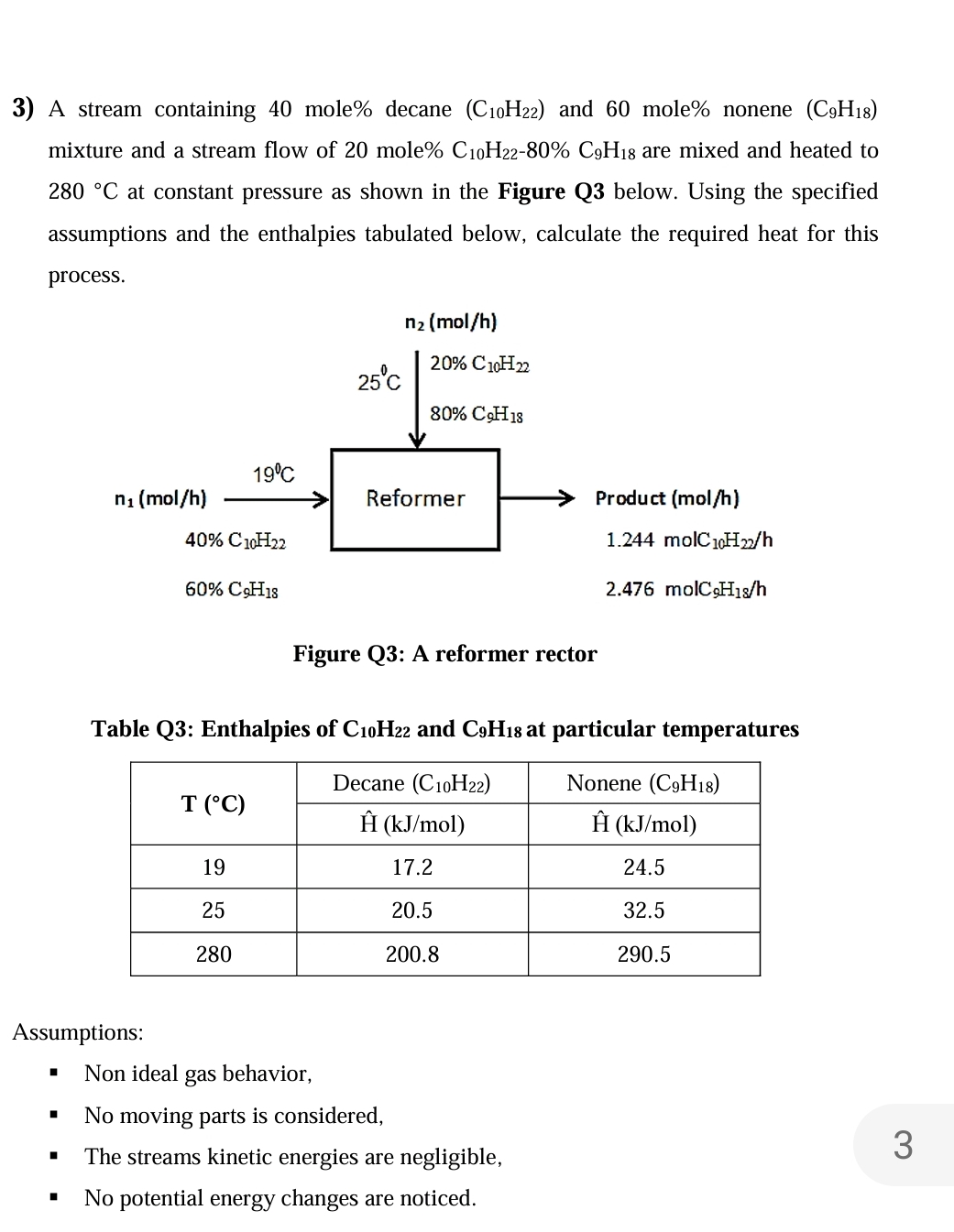
A stream containing mole decane and mole nonene mixture and a stream flow of mole are mixed and heated to at constant pressure as shown in the Figure Q below. Using the specified assumptions and the enthalpies tabulated below, calculate the required heat for this process.
Figure Q: A reformer rector
Table Q: Enthalpies of and at particular temperatures
tableDecane Nonene
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


