Question: A sugar molecule (Cat...) con diffuse freely in water at a certain temperature. A toy model below shows the super molecules movement with respect to
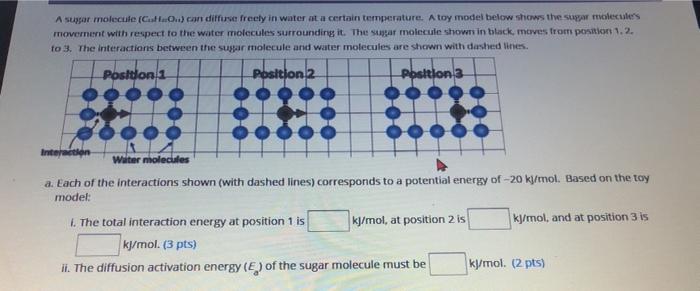
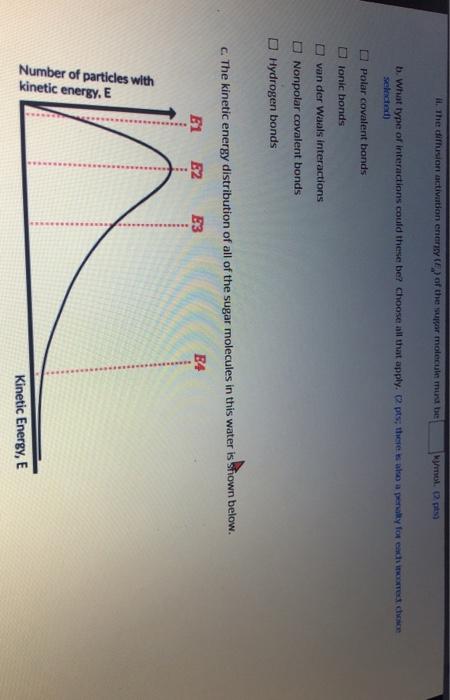
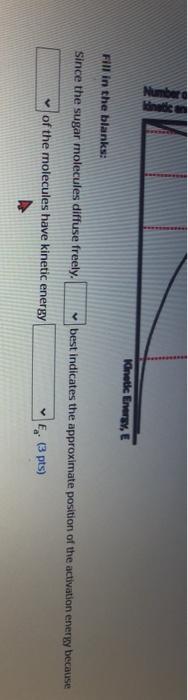
A sugar molecule (Cat...) con diffuse freely in water at a certain temperature. A toy model below shows the super molecules movement with respect to the water molecules surrounding it. The sugar molecule shown in black moves from portion 1.2. to 3. The interactions between the sugar molecule and water molecules are shown with dashed lines. Posidonia Position 2 Position 3 Interaction Water molecules a. Each of the interactions shown (with dashed lines) corresponds to a potential energy of -20 kJ/mol. Based on the toy model: 1. The total interaction energy at position 1 is kJ/mol, at position 2 is kj/mol, and at position 3 is kj/mol. (3 pts) II. The diffusion activation energy (E) of the sugar molecule must be kj/mol. (2 pts) I the diffusion activation enery of the super molecule mun be mol 02 tsa b. What type of interactions could these be? Choose all that apply. pts; theres ao a penalty for each one chce selected) Polar covalent bonds tonic bonds O van der Waals Interactions Nonpolar covalent bonds Hydrogen bonds c. The kinetic energy distribution of all of the sugar molecules in this water is shown below. E1 B2 E3 E4 Number of particles with kinetic energy. E Kinetic Energy, E Number Kinetic Energy, E Fill in the blanks: Since the sugar molecules diffuse freely. best indicates the approximate position of the activation energy because of the molecules have kinetic energy (3 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


