Question: A system is initially in an equilibrium state. It undergoes a process where the pressure decreases. What is true about the initial pressure, Pi ,
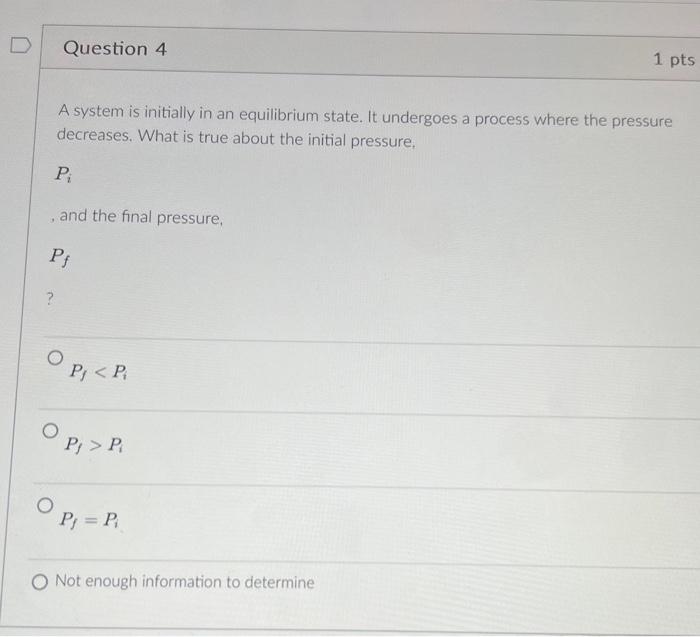

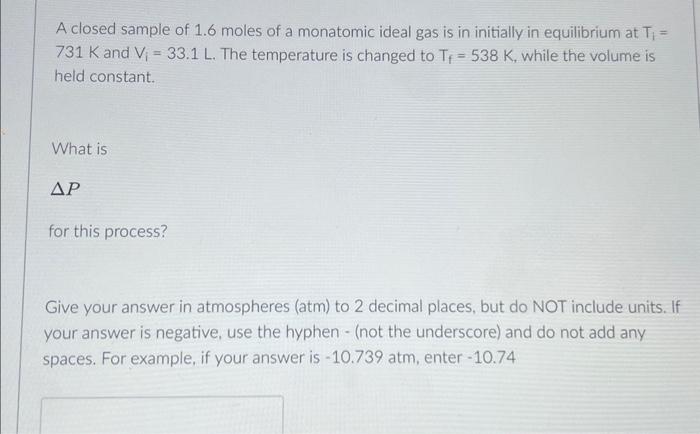
A system is initially in an equilibrium state. It undergoes a process where the pressure decreases. What is true about the initial pressure, Pi , and the final pressure, Pf Pf
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


