Question: A titration has 4 main areas (start titration, during titration, equivalence point, and after the equivalence point. Answer the question based on these main four
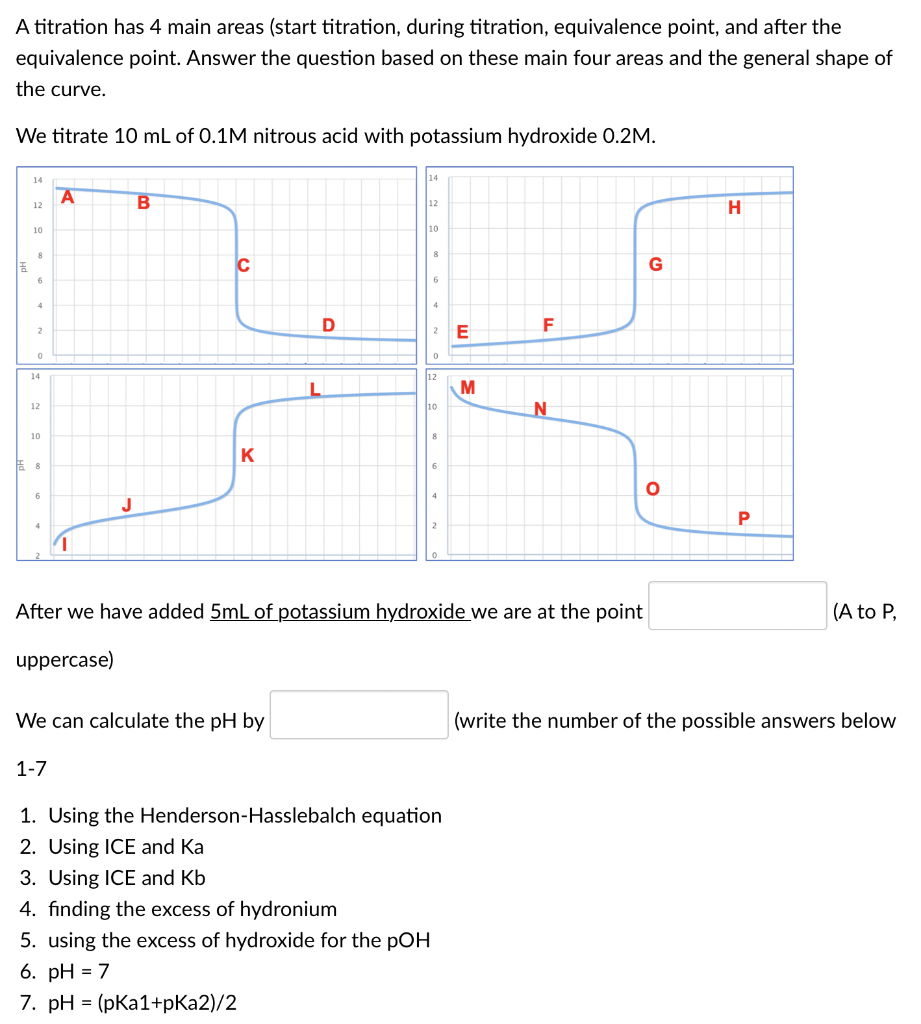
A titration has 4 main areas (start titration, during titration, equivalence point, and after the equivalence point. Answer the question based on these main four areas and the general shape of the curve. We titrate 10mL of 0.1M nitrous acid with potassium hydroxide 0.2M. After we have added 5mL of potassium hydroxide we are at the point (A to P uppercase) We can calculate the pH by (write the number of the possible answers below 1-7 1. Using the Henderson-Hasslebalch equation 2. Using ICE and Ka 3. Using ICE and Kb 4. finding the excess of hydronium 5. using the excess of hydroxide for the pOH 6. pH=7 7. pH=(pKa1+pKa2)/2
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


