Question: A: Water goes through the following two processes: 0.2000 m kg. Determine the internal 1a 2a Heating at constant pressure of 8.00 bar from

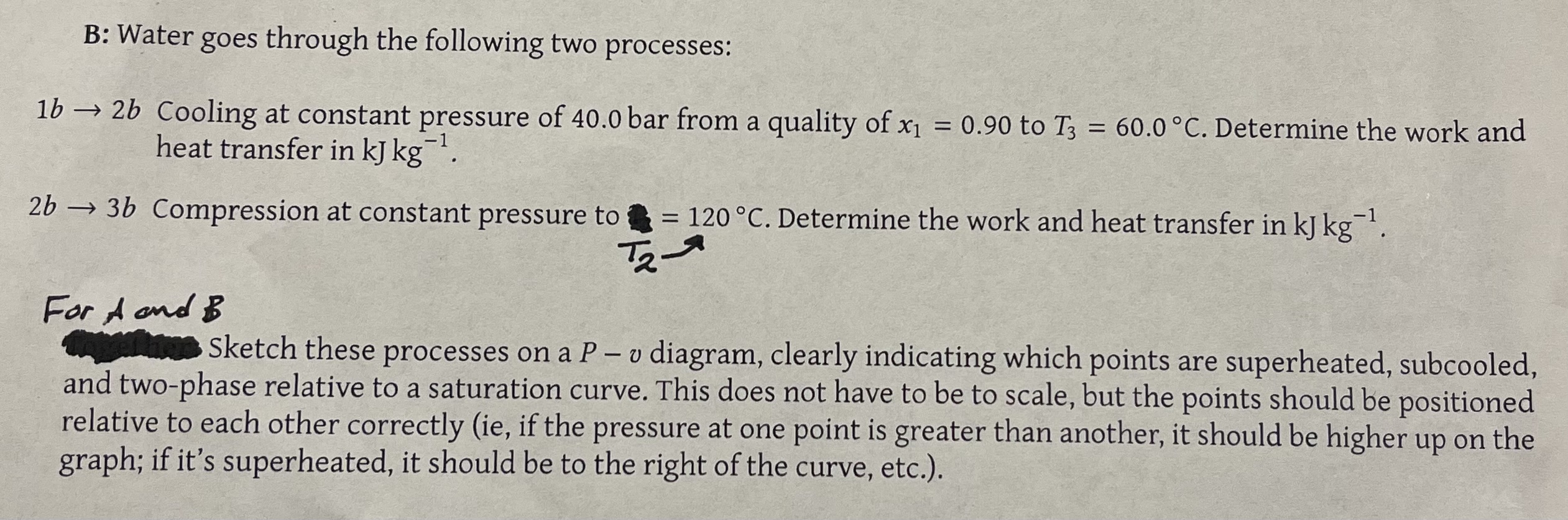
A: Water goes through the following two processes: 0.2000 m kg. Determine the internal 1a 2a Heating at constant pressure of 8.00 bar from saturated liquid. v2 = 0.2000 m kg energy, the heat transfer, and the work in kJ kg 2a 3a Heating at constant volume to saturated vapor. Determine the ending pressure in bar and the heat transfer in kJ kg 1. B: Water goes through the following two processes: x1 1b2b Cooling at constant pressure of 40.0 bar from a quality of x = 0.90 to T3 = 60.0 C. Determine the work and heat transfer in kJ kg. 2b3b Compression at constant pressure to = 120 C. Determine the work and heat transfer in kJ kg 1. For A and B T2 Sketch these processes on a P - v diagram, clearly indicating which points are superheated, subcooled, and two-phase relative to a saturation curve. This does not have to be to scale, but the points should be positioned relative to each other correctly (ie, if the pressure at one point is greater than another, it should be higher up on the graph; if it's superheated, it should be to the right of the curve, etc.).
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


