Question: A water molecule can be pictured as a triangle with O at one vertex and H at the other two. The angle HOH is 104
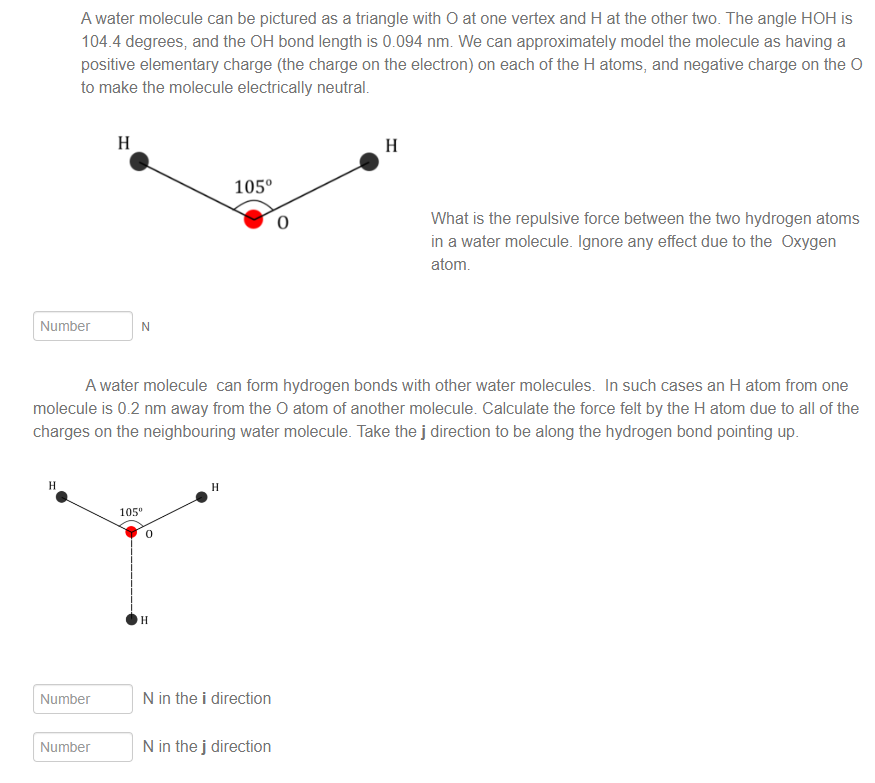
A water molecule can be pictured as a triangle with O at one vertex and H at the other two. The angle HOH is 104 4 degrees, and the OH bond length is 0.094 nm. We can approximately model the molecule as having a positive elementary charge (the charge on the electron) on each of the H atoms, and negative charge on the O to make the molecule electrically neutral. 105 0 What is the repulsive force between the two hydrogen atoms in a water molecule. Ignore any effect due to the Oxygen atom. Number N A water molecule can form hydrogen bonds with other water molecules. In such cases an H atom from one molecule is 0.2 nm away from the O atom of another molecule. Calculate the force felt by the H atom due to all of the charges on the neighbouring water molecule. Take the j direction to be along the hydrogen bond pointing up. H H 105" Q | I | | | @ Number N in the i direction Number N in the j direction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


