Question: a) Write the correct units for the reaction rate constant based on the descriptions given (8 points): i) 4th order overall ii) 2A + B
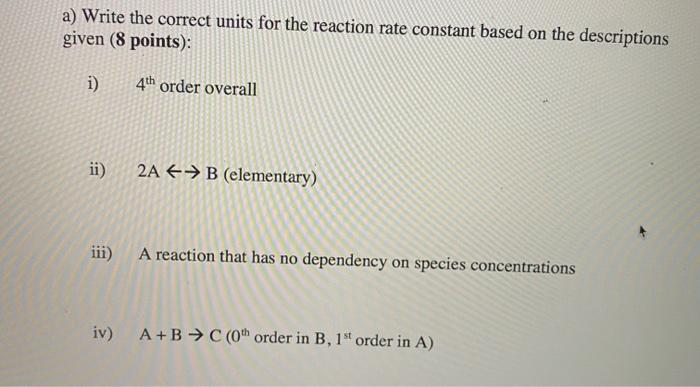



a) Write the correct units for the reaction rate constant based on the descriptions given (8 points): i) 4th order overall ii) 2A + B (elementary) iii) A reaction that has no dependency on species concentrations iv) A+B C (Oh order in B, 1 order in A) of 18 b) The frequency factor is a measure of the number of collisions that occur for a given mixture. (4 points) i) Discuss how and why a change in particle size changes the frequency factor. ii) Discuss how and why a change in system temperature changes the frequency factor. c) True or False? For the reaction A B, the rate of reaction for A continually decreases with decreasing amounts of A. (1 point) If the operating conditions of all reactors remain identical and constant, the conversion of two smaller PFRs in series will be the same as the conversion in a single larger PFR assuming the total volume of the two smaller PFRs is equal to the volume in the larger PFR. d) True or False? For the reaction A B, the rate of reaction for A continually decreases with decreasing amounts of A. (1 point) If the operating conditions of all reactors remain identical and constant, the size of a PFR needed to achieve X% conversion will ALWAYS be smaller than the volume of a CSTR needed to achieve the same conversion. e) The general mole balance can be simplified based on the type of reactor. Identify what terms cancel out of the mole balance equation and why for the following reactor descriptions. (6 points) i) Batch Reactor ii) CSTR iii) PFR
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


