Question: A,B,C, and D please What is the composition, in atom percent, of an alloy that consists of 4.5wt%Pb and 95.5 wt o Sn? The atomic
A,B,C, and D please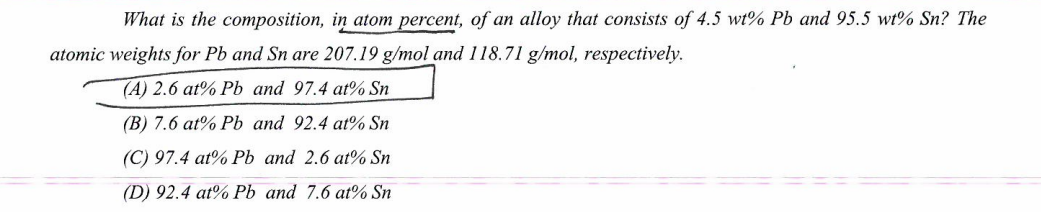
What is the composition, in atom percent, of an alloy that consists of 4.5wt%Pb and 95.5 wt o Sn? The atomic weights for Pb and Sn are 207.19g/mol and 118.71g/mol, respectively. (A) 2.6at%Pb and 97.4at%Sn (B) 7.6at%Pb and 92.4at%Sn (C) 97.4at%Pb and 2.6at%Sn (D) 92.4at%Pb and 7.6at%Sn
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


