Question: A pure sample of solid benzoic acid (CzH6O2) weighing 1.221 g was placed in a constant-volume bomb calorimeter and burned in an oxygen atmosphere.
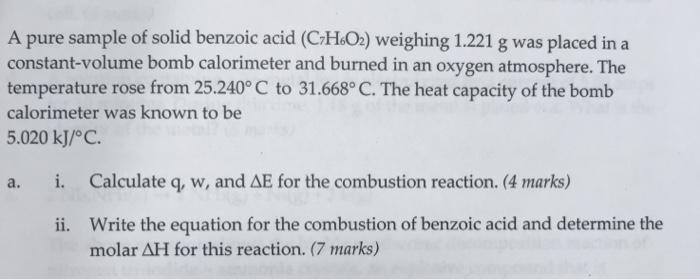
A pure sample of solid benzoic acid (CzH6O2) weighing 1.221 g was placed in a constant-volume bomb calorimeter and burned in an oxygen atmosphere. The temperature rose from 25.240 C to 31.668 C. The heat capacity of the bomb calorimeter was known to be 5.020 kJ/C. a. i. Calculate q, w, and AE for the combustion reaction. (4 marks) ii. Write the equation for the combustion of benzoic acid and determine the molar AH for this reaction. (7 marks)
Step by Step Solution
3.38 Rating (160 Votes )
There are 3 Steps involved in it
A pure sample of benzoic acid 74602 weiging 1221g was placed in ... View full answer

Get step-by-step solutions from verified subject matter experts


