Question: Acid - base titration 1 3 - 2 5 . 0 m L of sulfuric acid H 2 S O 4 solution was titrated with
Acidbase titration
of sulfuric acid solution was titrated with of MNaOH solution to produce Calculate the concentration of sulfuric acid in molL of solution Molar mass of
NaOH
a
b
c
d
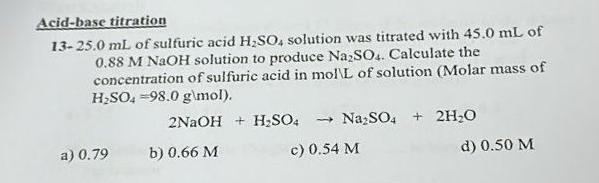
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


