Question: Please focus, I only need questions 4, 5 and 6 (I have already solved the rest so I don't need them) Experiment 4: Acid Base
Please focus, I only need questions 4, 5 and 6 (I have already solved the rest so I don't need them)

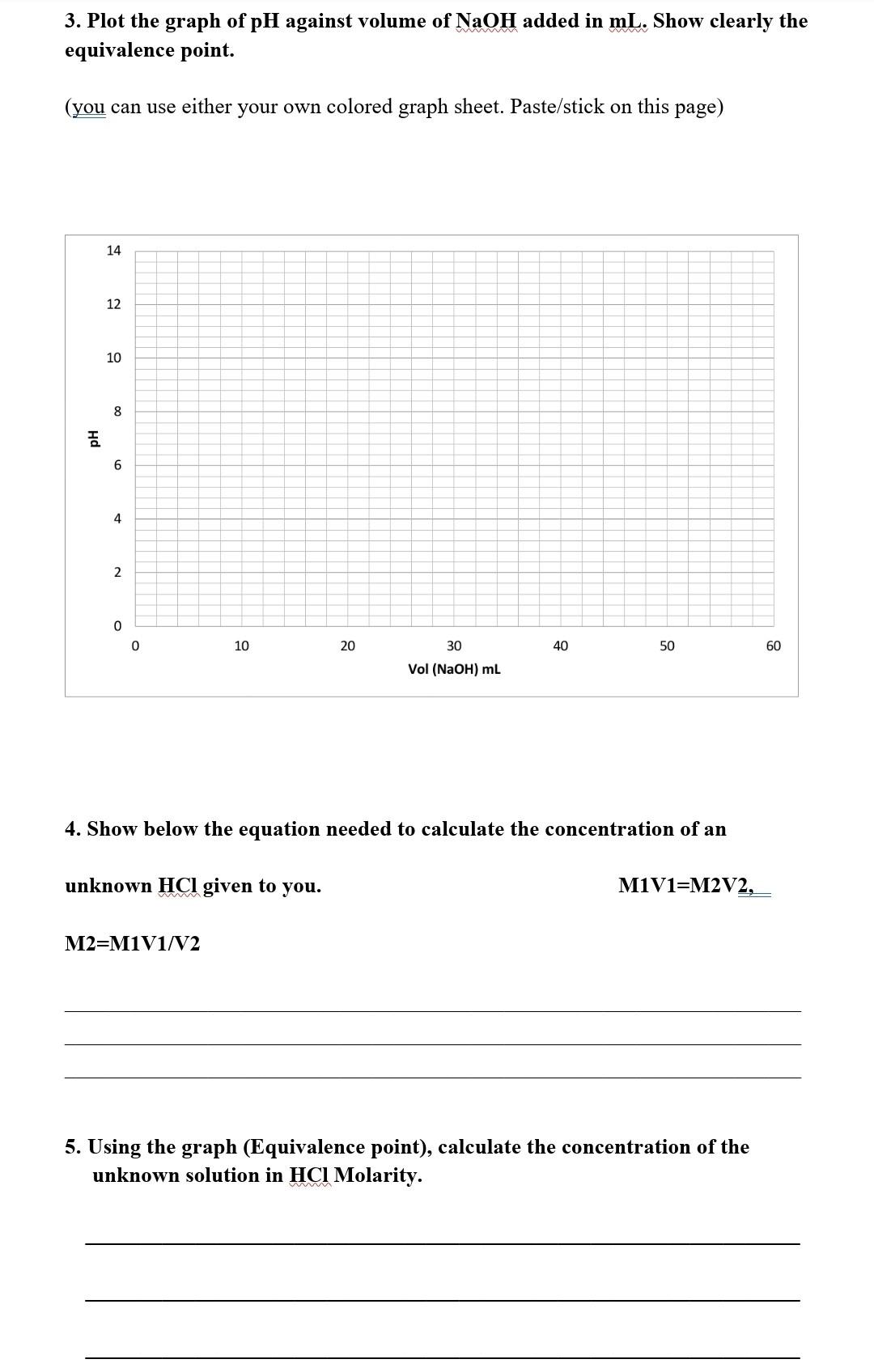

Experiment 4: Acid Base Titration Using a pH Meter and Phenolphthalein Strong acid vs Strong base Aim: To determine the concentration of an unknown solution of Hydrochloric acid, HCI (x M) using a given standard solution of 0.1M NaOH as a titrant. Using 2 Methods a. pH meter b. Classical technique using an indicator phenolphthalein. Procedure (summarized) 1. Pipette out 25mL of the unknown HCl x M acid solution into a 100mL beaker. 2. Calibrate the pH-meter with the given buffer 4 and buffer 7 solutions. 3. Fill the burette with 0.1 M NaOH solution. 4. Titrate the NaOH solution against x M HCl solution. 1. A. Calculate the mass of NaOH needed to prepare 100 mL of 0.1M NaOH solution.(RMM:40) W1= 0.4240g W2= 0.0081g B. Calculate the actual concentration (M) of the NaOH you weighed. 2. Calibrate the pH Meter given to you using pH buffer of pH 4 and pH7. Titrate and record readings as explained in your procedure and complete the following table; Vol NaOH pH Reading Vol NaOH added mL 0 added mL pH Reading 1.3 23 5.1 5 1.3 24 5.7 10 1.5 25 15 30 1.7 2.5 11.2 11.3 11.5 18 35 19 2.8 40 11.8 20 3.5 45 11.8 21 3.9 50 11.8 22 4.8 3. Plot the graph of pH against volume of NaOH added in mL. Show clearly the equivalence point. (you can use either your own colored graph sheet. Paste/stick on this page) 14 12 10 OC 8 6 4 2 0 0 10 20 30 40 50 60 Vol (NaOH) mL 4. Show below the equation needed to calculate the concentration of an unknown HCl given to you. M1V1=M2V2, M2=M1V1/V2 5. Using the graph (Equivalence point), calculate the concentration of the unknown solution in HCl Molarity. 6. a. Classical technique, titration of xHCl (strong acid) 25ml using 0.1MNaOH (strong base) and phenolphthalein as an indicator to determine the concentration of HCI. Initial Volume of NaOH 0.0ml Final Volume of NaOH= 25.2ml w Volume of NaOH used = w Molarity of HCI b. Compare the data obtained from pH meter and the classical technique. Concentration by classical method= Concentration by PH meter
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


