Question: 7. An adiabatic packed bed reactor is to be used for the oxidation of sulphur dioxide. The reactor consists of three beds, bed 1


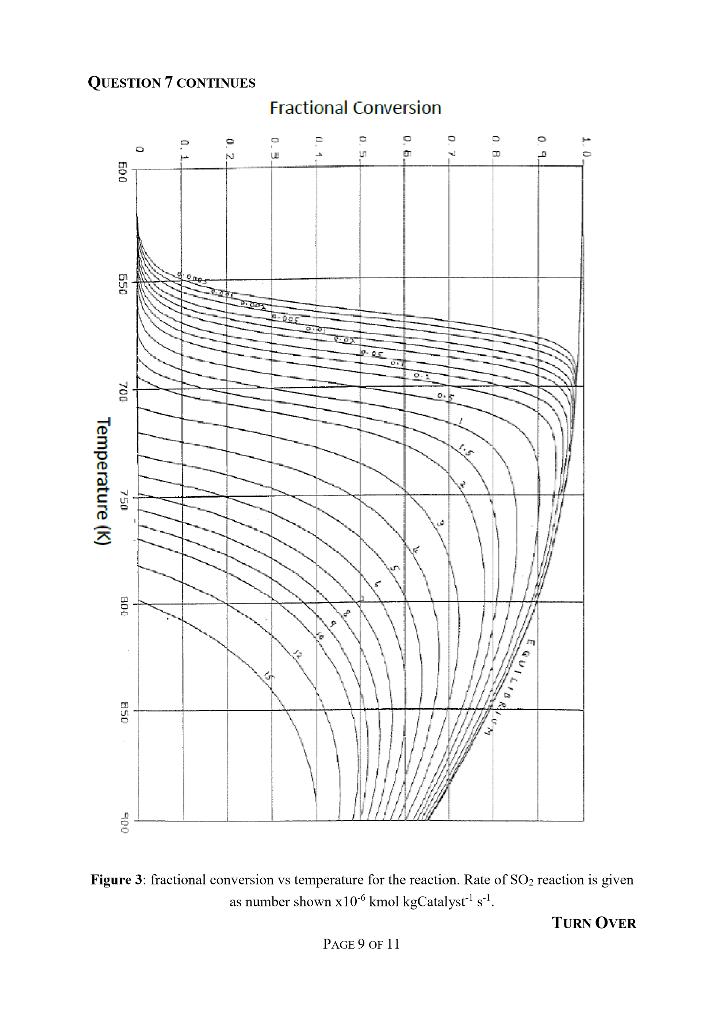
7. An adiabatic packed bed reactor is to be used for the oxidation of sulphur dioxide. The reactor consists of three beds, bed 1 contains 15 tonnes of catalyst and beds 2 and 3 contains 5 tonnes of catalyst each. Between each subsequent bed is an external heat exchanger, prior to bed 2 the reaction mixture is brought to 750 K and prior to bed 3 the reaction mixture is brought to 700 K. The feed gas is available at 200 C, but is pre- heated to the reactor inlet temperature of 377 C by counter-current heat exchange with the exhaust gases. The composition of the feed gas is SO - 12.7 kmol, O = 8.6 kmol and N = 78.7 kmol. Data: The reaction occurring is: SO +0.50 SO The reaction enthalpy at the reactor inlet temperature is -93,000 kJ/kmole. Mean molar heat capacities for the species over the range 400 to 600 C are: SO = 51.1 kJ kmol- K, O = 33.1 kJ kmol K-, N = 30.1 kJ kmol K- and SOs = 75.8 kJ kmol K'. A fractional conversion vs temperature plot for the reaction is available on the next page as Figure 3. (b) Draw a simple diagram of the reactor set-up illustrating the general structure, flow direction and temperatures expected. [3 marks] If the first catalytic bed achieves equilibrium, the second bed achieves fractional conversion of 0.8 and the final bed achieves equilibrium. What will be the temperature at the exit of the first bed, the fractional conversion at the exit of the first and third beds? [10 marks] (c) Comment on any assumptions made. QUESTION 7 CONTINUES ON THE NEXT PAGE PAGE 8 OF 11 [2 marks] [Total 15 marks] TURN OVER QUESTION 7 CONTINUES Temperature (K) 650 53 C N Fractional Conversion Figure 3: fractional conversion vs temperature for the reaction. Rate of SO reaction is given as number shown x10 kmol kgCatalyst s. PAGE 9 OF 11 TURN OVER
Step by Step Solution
3.54 Rating (185 Votes )
There are 3 Steps involved in it
To solve the given problem we need to approach each part systematically a Drawing the Reactor Setup ... View full answer

Get step-by-step solutions from verified subject matter experts


