Question: After completing this experiment, the student should be able to: 1. Demonstrate the concept of quantitative analysis. 2. Make solution and standardize it. 3.
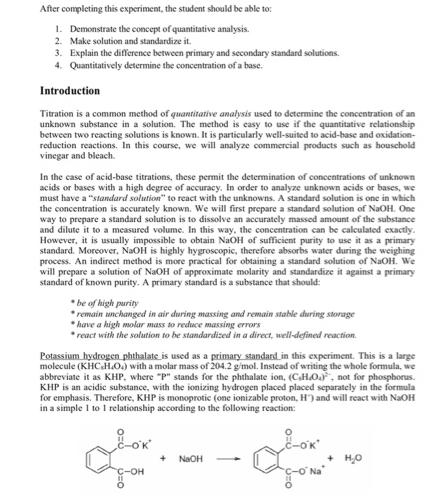
After completing this experiment, the student should be able to: 1. Demonstrate the concept of quantitative analysis. 2. Make solution and standardize it. 3. Explain the difference between primary and secondary standard solutions. 4. Quantitatively determine the concentration of a base. Introduction Titration is a common method of quantitative analysis used to determine the concentration of an unknown substance in a solution. The method is easy to use if the quantitative relationship between two reacting solutions is known. It is particularly well-suited to acid-base and oxidation- reduction reactions. In this course, we will analyze commercial products such as household vinegar and bleach. In the case of acid-base titrations, these permit the determination of concentrations of unknown acids or bases with a high degree of accuracy. In order to analyze unknown acids or bases, we must have a "standard solution" to react with the unknowns. A standard solution is one in which the concentration is accurately known. We will first prepare a standard solution of NaOH. One way to prepare a standard solution is to dissolve an accurately massed amount of the substance and dilute it to a measured volume. In this way, the concentration can be calculated exactly. However, it is usually impossible to obtain NaOH of sufficient purity to use it as a primary standard. Moreover, NaOH is highly hygroscopic, therefore absorbs water during the weighing process. An indirect method is more practical for obtaining a standard solution of NaOH. We will prepare a solution of NaOH of approximate molarity and standardize it against a primary standard of known purity. A primary standard is a substance that should: be of high purity remain unchanged in air during massing and remain stable during storage *have a high molar mass to reduce massing errors react with the solution to be standardized in a direct, well-defined reaction. Potassium hydrogen phthalate is used as a primary standard in this experiment. This is a large molecule (KHC.H.O.) with a molar mass of 204.2 g/mol. Instead of writing the whole formula, we abbreviate it as KHP, where "P" stands for the phthalate ion, (C.H.O.), not for phosphorus. KHP is an acidic substance, with the ionizing hydrogen placed placed separately in the formula for emphasis. Therefore, KHP is monoprotic (one ionizable proton, H') and will react with NaOH in a simple 1 to 1 relationship according to the following reaction: Llow. + NaOH C-O + HO In this experiment, phenolphthalein will be used as an indicator which helps in determining endpoint and sodium hydroxide will be standardized. Safety Precautions Wear safety goggles while working in the laboratory. NaOH is corrosive so avoid skin contact. Follow all safety rules described in experiment 1. Materials and Equipment Buret (50 mL), beaker (200 mL), graduated cylinder. Erlenmeyer flask (250 mL), funnel, balance (10 mg or 1 mg accuracy), solid potassium hydrogen phthalate (KHP), NaOH Procedure You will be provided NaOH with approximate Molarity to use between 20 and 40 ml. in the titration of 0.8 g KHP. 1. Accurately weigh about 0.8 g KHP (when using 50 mL buret) into a 250 mL Erlenmeyer flask. Add about 50 mL of water and swirl the flask until the sample is dissolved. Add 3 drops of phenolphthalein indicator (colorless in acidic solution; pink in basic solution). 2. Titrate the KHP solution with NaOH solution to be standardized. Buret readings should be to two decimal places, with the second decimal place estimated between the two lines. The titration should proceed until the faintest pink color persists for 30 sec. after mixing. The color will fade upon exposure to the air (WHY?). After completing a trial, breathe into the flask and swirl. What has happened? 3. Repeat the titration three times (this is called doing the measurement in triplicate). For accurate work, the three molarities must be within 1% of one another. Then calculate the average molarity, M, of the NaOH. 62 Experimental General Chemistry 1 Laboratory Data Sheet: Name: Show all calculations and results with correct significant figures! 2 decimal places for buret volumes! Trial I 2 re Mass of KHP () Experiment 12: Standardization of NaOH Solution Section: Average Molarity of NaOH (1) Moles of KHP (mole) V, (nt.) Calculations: V: (ml) Von (ml)-V-V, moles NaOH Mo-NaOH) Molaverage) (M. M.M.33, and the standard deviation SD- V (L) Calculations (show all calculations next parel: V, - buret reading before. V-buret reading after. V-V-V Report two decimal places for V, V and V and at least three significant figures for Mo Thus, volumes should be recorded eg as V-21.36 mL or 18.50 ml.. (not 18.5) %50- Moles of KHP-mass of KHP/molar mass of KHP, then moles KHP-noles NaOH (KHP is monoprotic). Show your calculation for trial 1 Molarity of NaOH (mol/L) SD- mol/L Repeat this calculation for each trial, then calculate the average Mon from your 3 best trials AM-M %SD (SD/M 100% 63
Step by Step Solution
3.49 Rating (149 Votes )
There are 3 Steps involved in it
To calculate the molarity of the NaOH solution after standardization follow these steps for each tri... View full answer

Get step-by-step solutions from verified subject matter experts




