Question: Compute the unit melting energy for (a) aluminum and (b) steel as the sum of: (1) the heat required to raise the temperature of
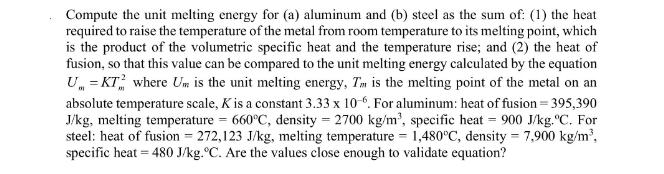
Compute the unit melting energy for (a) aluminum and (b) steel as the sum of: (1) the heat required to raise the temperature of the metal from room temperature to its melting point, which is the product of the volumetric specific heat and the temperature rise; and (2) the heat of fusion, so that this value can be compared to the unit melting energy calculated by the equation U = KT where Um is the unit melting energy, Tm is the melting point of the metal on an absolute temperature scale, K is a constant 3.33 x 10-6. For aluminum: heat of fusion=395,390 J/kg, melting temperature = 660C, density = 2700 kg/m, specific heat = 900 J/kg. "C. For steel: heat of fusion = 272,123 J/kg, melting temperature = 1,480C, density = 7,900 kg/m, specific heat = 480 J/kg. C. Are the values close enough to validate equation?
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it
To compute the unit melting energy for aluminum and steel we need to consider two components 1 Energ... View full answer

Get step-by-step solutions from verified subject matter experts


