Question: All problems in this assignment concern the non-elementary, liquid-phase production of propylene glycol (PG) by hydrolysis of propylene oxide (PO) with water (W) in
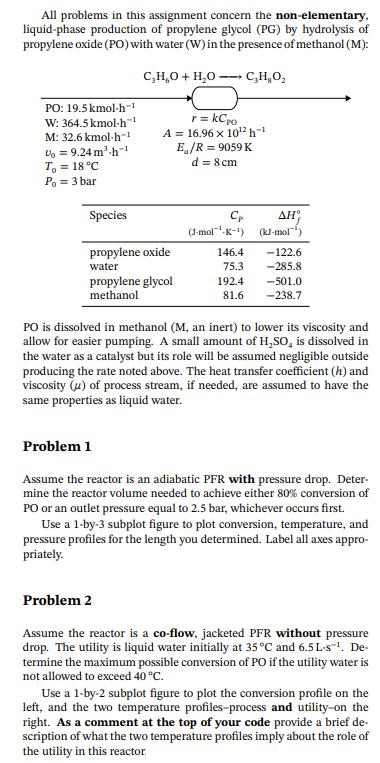
All problems in this assignment concern the non-elementary, liquid-phase production of propylene glycol (PG) by hydrolysis of propylene oxide (PO) with water (W) in the presence of methanol (M): PO: 19.5 kmol-h W: 364.5 kmol-h M: 32.6 kmol-h-1 U = 9.24m-h T = 18C Po=3 bar C,H,O + HO r = kCpo C,HO A 16.96 x 102h-1 E/R = 9059 K d = 8cm Species (J-mol-K-) (kJ-mol propylene oxide 146.4 -122.6 water 75.3 -285.8 propylene glycol methanol 192.4 -501.0 81.6 -238.7 PO is dissolved in methanol (M, an inert) to lower its viscosity and allow for easier pumping. A small amount of HSO, is dissolved in the water as a catalyst but its role will be assumed negligible outside producing the rate noted above. The heat transfer coefficient (h) and viscosity (u) of process stream, if needed, are assumed to have the same properties as liquid water. Problem 1 Assume the reactor is an adiabatic PFR with pressure drop. Deter- mine the reactor volume needed to achieve either 80% conversion of PO or an outlet pressure equal to 2.5 bar, whichever occurs first. Use a 1-by-3 subplot figure to plot conversion, temperature, and pressure profiles for the length you determined. Label all axes appro- priately. Problem 2 Assume the reactor is a co-flow, jacketed PFR without pressure drop. The utility is liquid water initially at 35 C and 6.5 L-s. De- termine the maximum possible conversion of PO if the utility water is not allowed to exceed 40 C. Use a 1-by-2 subplot figure to plot the conversion profile on the left, and the two temperature profiles-process and utility-on the right. As a comment at the top of your code provide a brief de- scription of what the two temperature profiles imply about the role of the utility in this reactor.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


