Question: Please solve Exercise 4.8 in the image below: 4.10 Exercises 177 olvent, d with less centrat Exercise 4.8: Nonconstant density with a liquid-phase reaction Propylene
Please solve Exercise 4.8 in the image below:
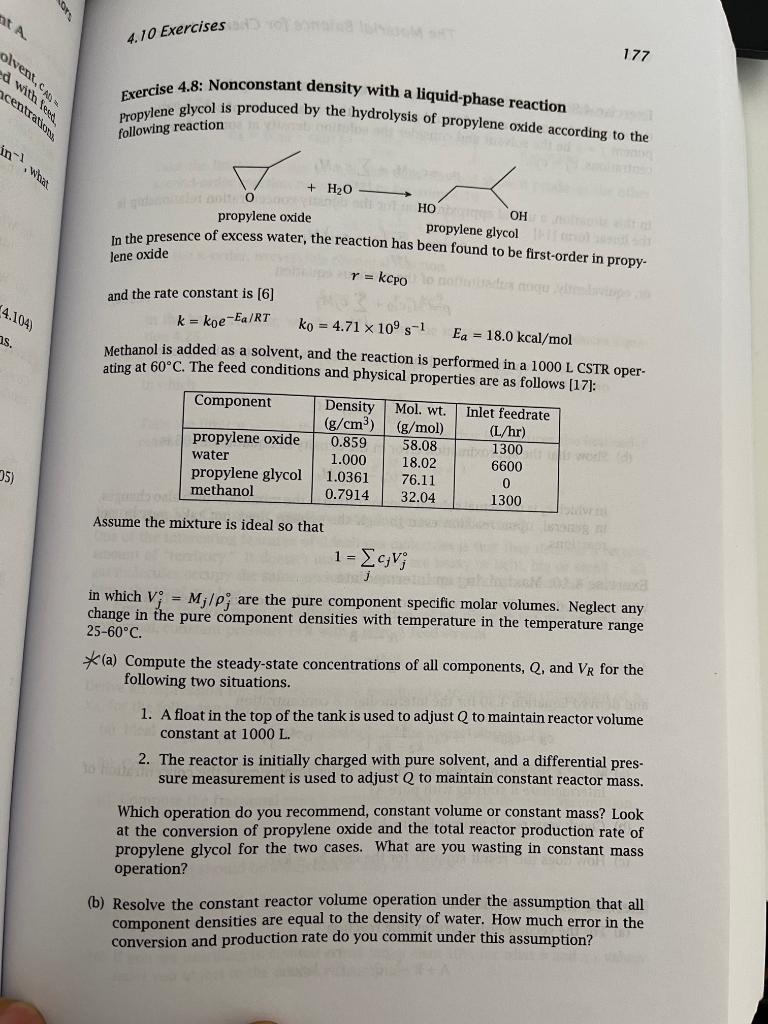
4.10 Exercises 177 olvent, d with less centrat Exercise 4.8: Nonconstant density with a liquid-phase reaction Propylene glycol is produced by the hydrolysis of propylene oxide according to the following reaction int What lene oxide 4.104 s. 5) + H2O no HO OH propylene oxide propylene glycol in the presence of excess water, the reaction has been found to be first-order in propy- r = kcpoort and the rate constant is [6] k = koe-Ea/RT ko = 4.71 x 10's-1 Ea = 18.0 kcal/mol Methanol is added as a solvent, and the reaction is performed in a 1000 L CSTR oper- ating at 60C. The feed conditions and physical properties are as follows (17): Component Density Mol. wt. Inlet feedrate (g/cm3) (g/mol) (L/hr) propylene oxide 0.859 58.08 1300 water 1.000 18.02 6600 propylene glycol 1.0361 76.11 methanol 0.7914 32.04 1300 Assume the mixture is ideal so that 1 = c;V; j in which V; = M;/pare the pure component specific molar volumes. Neglect any change in the pure component densities with temperature in the temperature range 25-60C. *(a) Compute the steady-state concentrations of all components, Q, and VR for the following two situations. 1. A float in the top of the tank is used to adjust Q to maintain reactor volume constant at 1000 L. 2. The reactor is initially charged with pure solvent, and a differential pres- sure measurement is used to adjust to maintain constant reactor mass. 0 Which operation do you recommend, constant volume or constant mass? Look at the conversion of propylene oxide and the total reactor production rate of propylene glycol for the two cases. What are you wasting in constant mass operation? (b) Resolve the constant reactor volume operation under the assumption that all component densities are equal to the density of water. How much error in the conversion and production rate do you commit under this assumption
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


