Question: An aqueous potassium iodate stock solution is made by dissolving 7.18 mol KIO, in sufficient water for the final volume of the solution to be
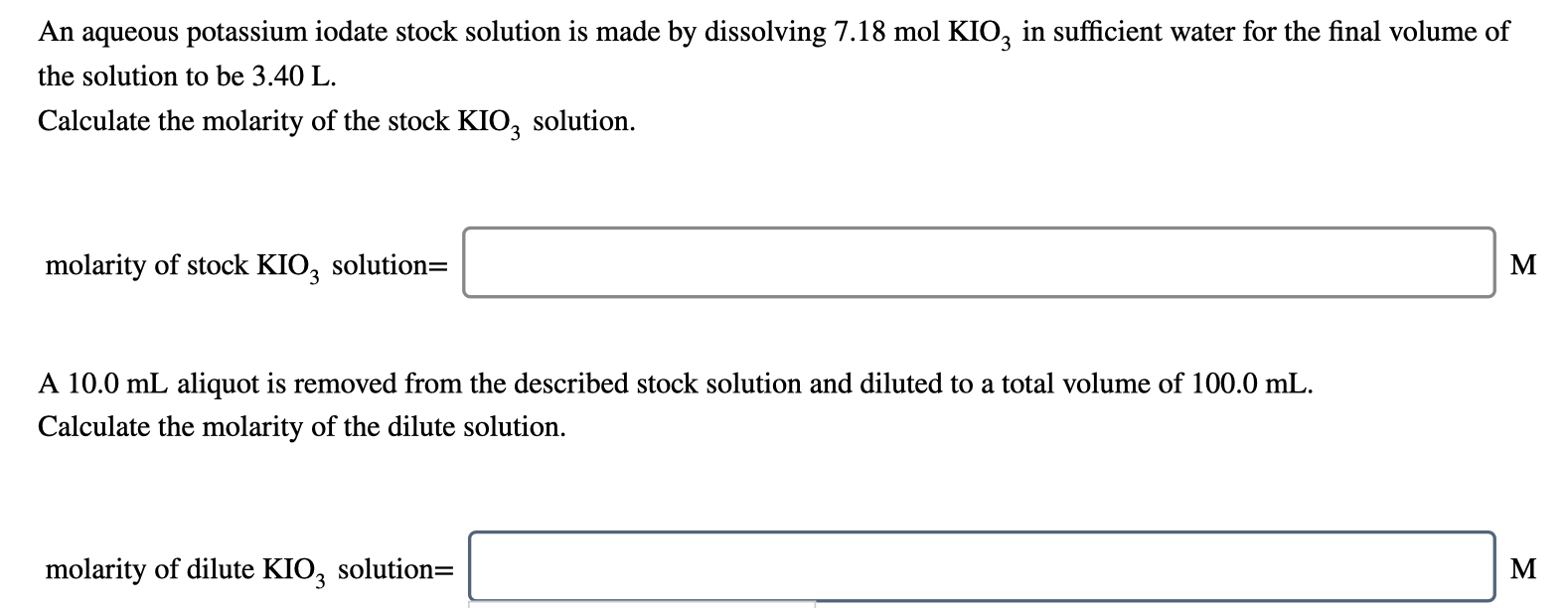
An aqueous potassium iodate stock solution is made by dissolving 7.18 mol KIO, in sufficient water for the final volume of the solution to be 3.40 L. Calculate the molarity of the stock KIO3 solution. molarity of stock KIO2 solution= M A 10.0 mL aliquot is removed from the described stock solution and diluted to a total volume of 100.0 mL. Calculate the molarity of the dilute solution. molarity of dilute KIO3 solution= M
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


