Question: please answer all Consider the neutralization reaction 2 HNO, (aq) + Ba(OH), (aq) + 2H2O(l) + Ba(NO),() A 0.105 L sample of an unknown HNO,
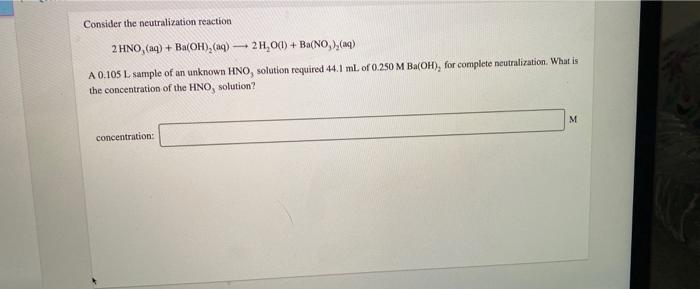
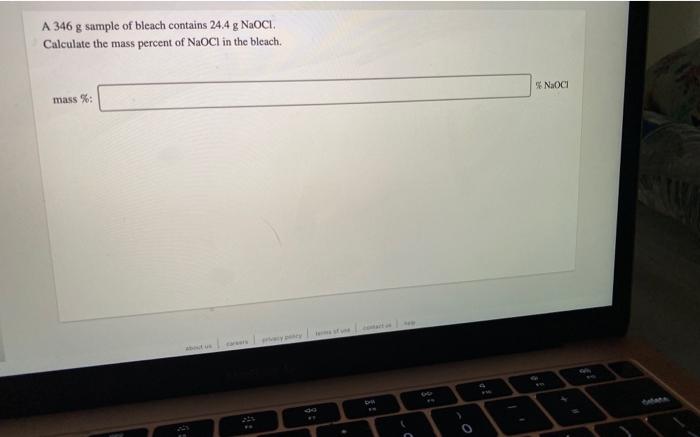
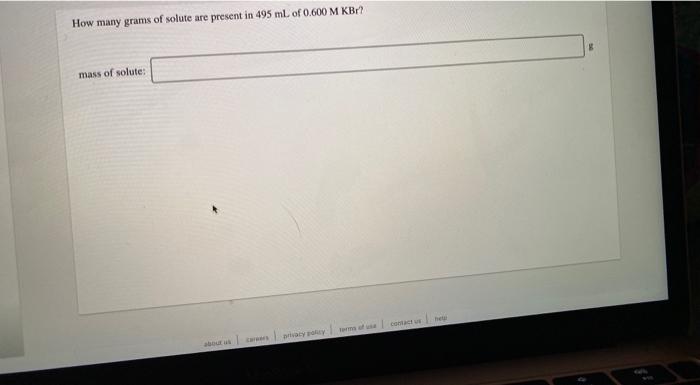
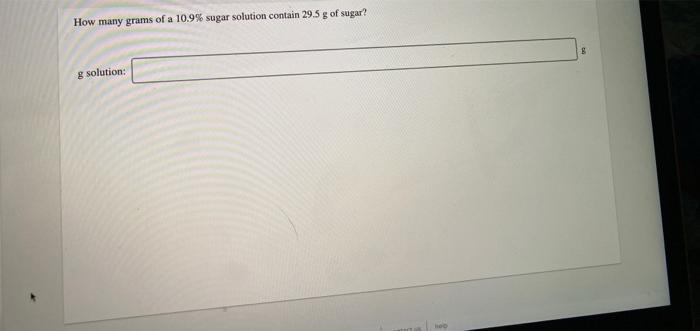

Consider the neutralization reaction 2 HNO, (aq) + Ba(OH), (aq) + 2H2O(l) + Ba(NO),() A 0.105 L sample of an unknown HNO, solution required 44.1 ml. of 0.250 M Ba(OH), for complete neutralization. What is the concentration of the HNO, solution? M concentration: A 346 g sample of bleach contains 24.4 g NaOCI. Calculate the mass percent of NaCl in the bleach. Noc mass %: How many grams of solute are present in 495 ml of 0.600 M KBr? mass of solute: can Drama How many grams of a 10.9% sugar solution contain 29.5 g of sugar? 8 8 solution: An aqueous potassium iodate (KIO,) solution is made by dissolving 594 grams of KIO, in sufficient water so that the final volume of the solution is 4.00 L. Calculate the molarity of the KIO, solution. M [KIO, 1=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


