Question: An electric current is conducted through water, decomposing it into H and O2 gas, as shown in the reaction below. The reaction takes place
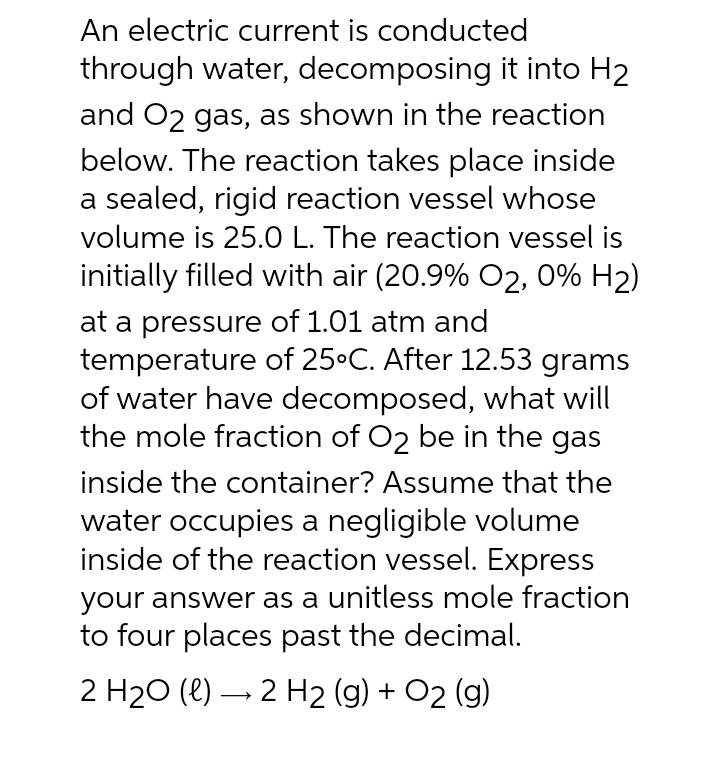
An electric current is conducted through water, decomposing it into H and O2 gas, as shown in the reaction below. The reaction takes place inside a sealed, rigid reaction vessel whose volume is 25.0 L. The reaction vessel is initially filled with air (20.9% O2, 0% H) at a pressure of 1.01 atm and temperature of 25C. After 12.53 grams of water have decomposed, what will the mole fraction of O2 be in the gas inside the container? Assume that the water occupies a negligible volume inside of the reaction vessel. Express your answer as a unitless mole fraction to four places past the decimal. 2 HO (l) 2 H2 (g) + O2 (g)
Step by Step Solution
3.58 Rating (158 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


