Question: An evaporation-crystallization process is used to obtain solid potassium sulfate from an aqueous solution of this salt. The fresh feed to the process (Stream 1
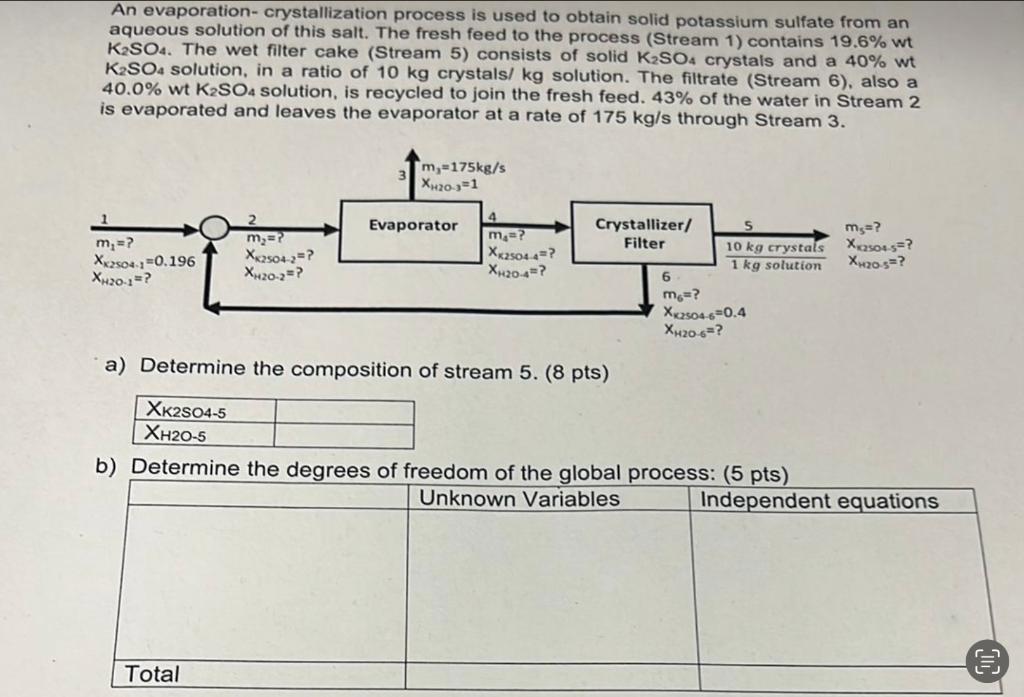
An evaporation-crystallization process is used to obtain solid potassium sulfate from an aqueous solution of this salt. The fresh feed to the process (Stream 1 ) contains 19.6%wt K2SO4. The wet filter cake (Stream 5) consists of solid K2SO4 crystals and a 40% wt K2SO4 solution, in a ratio of 10kg crystals/ kg solution. The filtrate (Stream 6), also a 40.0% wt K2SO4 solution, is recycled to join the fresh feed. 43% of the water in Stream 2 is evaporated and leaves the evaporator at a rate of 175kg/s through Stream 3 . a) Determine the composition of stream 5.(8pts) b) Determine the degrees of freedom of the alobal process: ( 5 bts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


