Question: Evaporation crystallization process is used to obtain solid potassium sulfate (K SO, S) from an aqueous solution of its salt. Knowing that the fresh foed
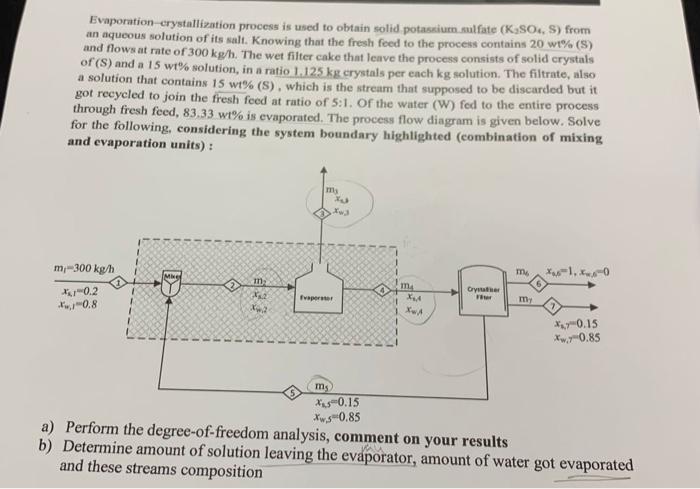
Evaporation crystallization process is used to obtain solid potassium sulfate (K SO, S) from an aqueous solution of its salt. Knowing that the fresh foed to the process contains 20 wt% (S) and flows at rate of 300 kg/h. The wet filter cake that leave the process consists of solid crystals of (S) and a 15 wt% solution, in a ratio 1.125 kg crystals per ench kg solution. The filtrate, also a solution that contains 15 wt% (S), which is the stream that supposed to be discarded but it got recycled to join the fresh feed at ratio of 5:1. of the water (W) fed to the entire process through fresh feed, 83,33 wt% is evaporated. The process flow diagram is given below. Solve for the following considering the system boundary highlighted (combination of mixing and evaporation units): mi m-300 kg/h IMINE my *.1.0 m m *10.2 -0.8 my 1 X4 * 0.15 *w 0.85 ms Xus0.15 *w.s0.85 a) Perform the degree-of-freedom analysis, comment on your results b) Determine amount of solution leaving the evaporator, amount of water got evaporated and these streams composition
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


