Question: Q1. An evaporation-crystallization process is used to obtain solid potassium sulfate from an aqueous solution of this salt. The fresh feed to the process contains
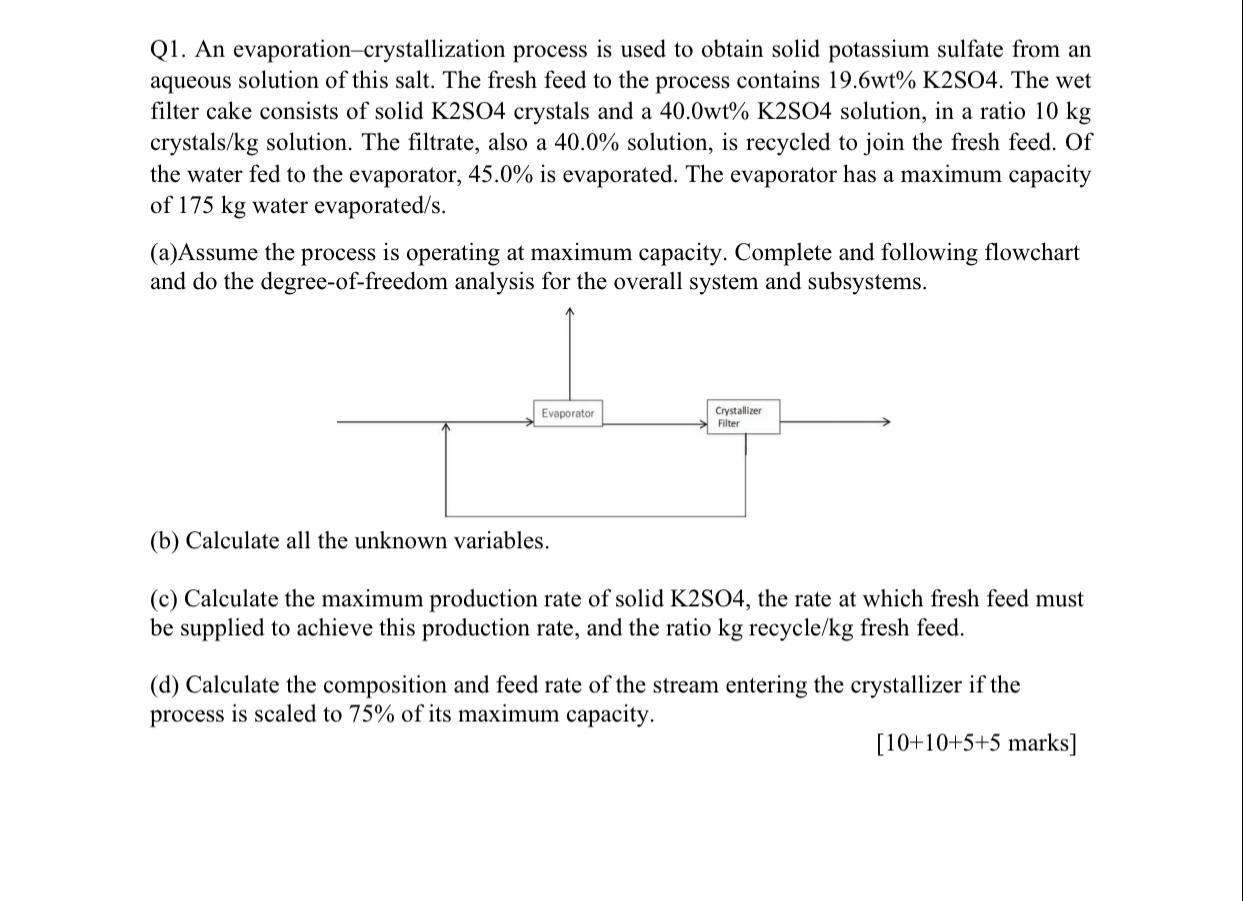
Q1. An evaporation-crystallization process is used to obtain solid potassium sulfate from an aqueous solution of this salt. The fresh feed to the process contains 19.6wt% K2504. The wet filter cake consists of solid K2SO4 crystals and a 40.0wt% K2SO4 solution, in a ratio 10 kg crystals/kg solution. The filtrate, also a 40.0% solution, is recycled to join the fresh feed. Of the water fed to the evaporator, 45.0% is evaporated. The evaporator has a maximum capacity of 175 kg water evaporated/s. (a)Assume the process is operating at maximum capacity. Complete and following flowchart and do the degree-of-freedom analysis for the overall system and subsystems. Evaporator Crystallizer Filter (b) Calculate all the unknown variables. (c) Calculate the maximum production rate of solid K2S04, the rate at which fresh feed must be supplied to achieve this production rate, and the ratio kg recycle/kg fresh feed. (d) Calculate the composition and feed rate of the stream entering the crystallizer if the process is scaled to 75% of its maximum capacity. [10+10+5+5 marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


