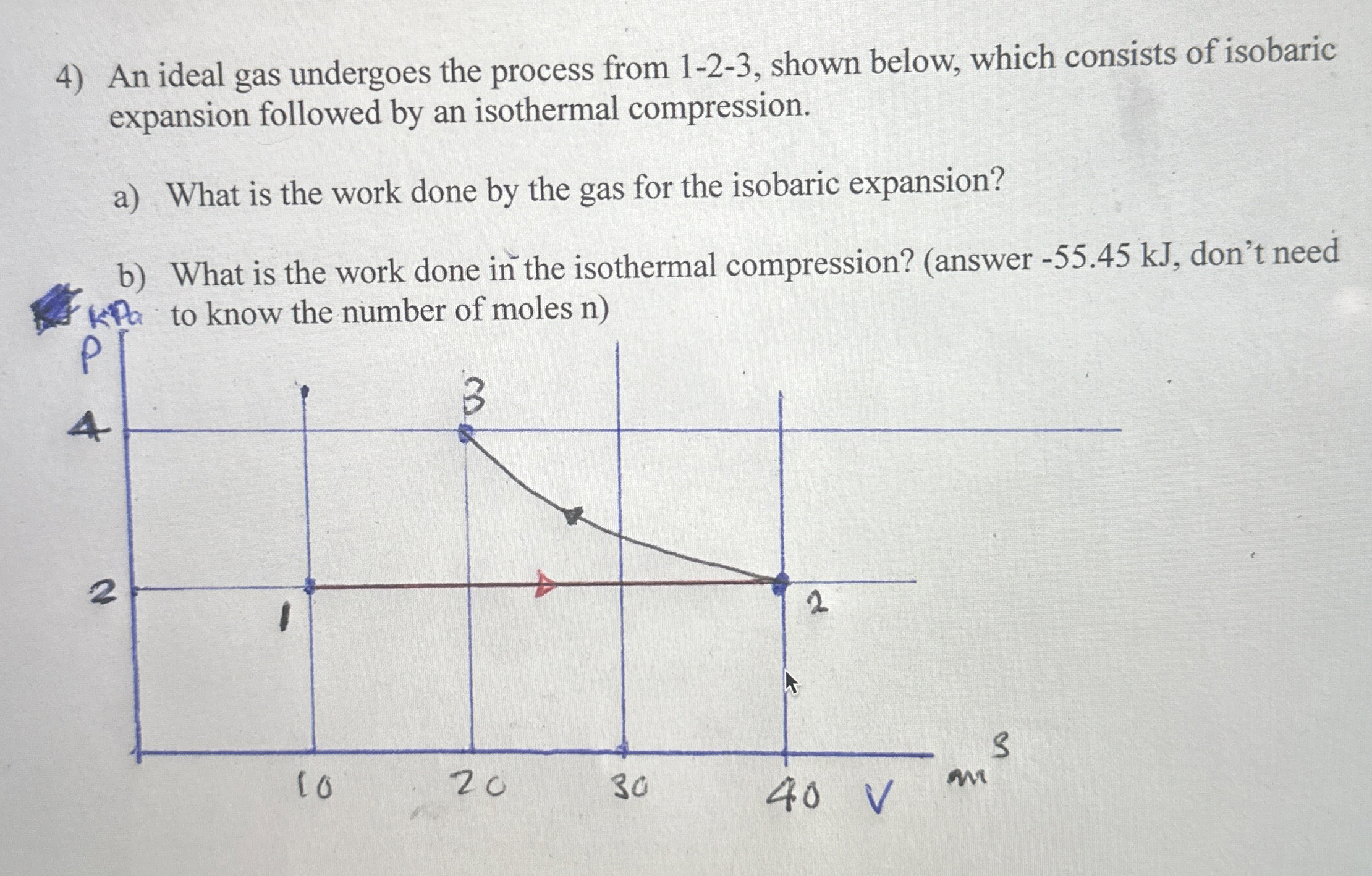
Question: An ideal gas undergoes the process from 1 - 2 - 3 , shown below, which consists of isobaric expansion followed by an isothermal compression.
An ideal gas undergoes the process from shown below, which consists of isobaric expansion followed by an isothermal compression.
a What is the work done by the gas for the isobaric expansion?
b What is the work done in the isothermal compression? answer kJ don't need to know the number of moles n
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


