Question: ANSWER ALL ASAP Consider this reaction, which occurs in the atmosphere and contributes to photochemical smog: H2(g)+I2(s)2HI(g) If there is 16.22gH2 and excess I2 present,
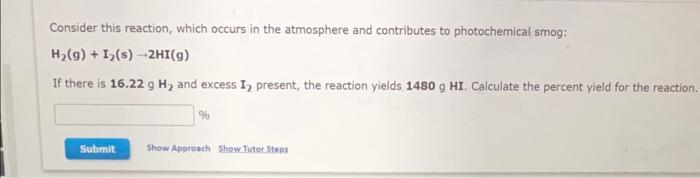
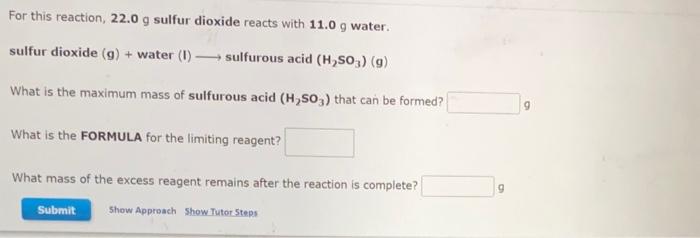

Consider this reaction, which occurs in the atmosphere and contributes to photochemical smog: H2(g)+I2(s)2HI(g) If there is 16.22gH2 and excess I2 present, the reaction yields 1480gHI. Calculate the percent yield for the reaction. % For this reaction, 22.0g sulfur dioxide reacts with 11.0g water. sulfur dioxide (g)+ water (I) sulfurous acid (H2SO3)(g) What is the maximum mass of sulfurous acid (H2SO3) that can be formed? 9 What is the FORMULA for the limiting reagent? What mass of the excess reagent remains after the reaction is complete? 9 Ascorbic acid, or vitamin C(C6H5O6), is an essential vitamin. It cannot be stored by the body and must be present in the diet. What is the molar mass of ascorbic acid? Vitamin C tablets are taken as a dietary supplement. If a typical tablet contains 523.0mg vitamin C, what amount (moles) and what number of molecules of vitamin C does it contain? Molar mass = g/mol molC6H8O6 molecules C6H8O6
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


