Question: answer ALL based on equation and quench reagent given; reaction was quenched with chosen base NaOH e. Which species will be present in the organic

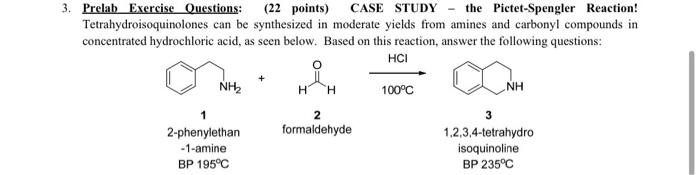
e. Which species will be present in the organic layer and the aqueous layer? Draw all species below. (2 points) Organic layer contains: Aqueous layer contains: f. After the quench and separation of layers, can you separate starting material (1) and product (3) using acid base extraction? Explain why or why not. (1 point) g. After a work-up, what type of distillation method would one use to separate the product (3) from trace amounts (less than 1%) of starting material (1)? Explain thoroughly, making sure to include the concept of theoretical plates in your answer. 3 points) 3. Prelab Exercise Questions: (22 points) CASE STUDY the Pictet-Spengler Reaction! Tetrahydroisoquinolones can be synthesized in moderate yields from amines and carbonyl compounds in concentrated hydrochloric acid, as seen below. Based on this reaction, answer the following questions: HCI + . NIL H H 100C NH 2 formaldehyde 2-phenylethan -1-amine BP 195C 3 1,2,3,4-tetrahydro isoquinoline BP 235C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


