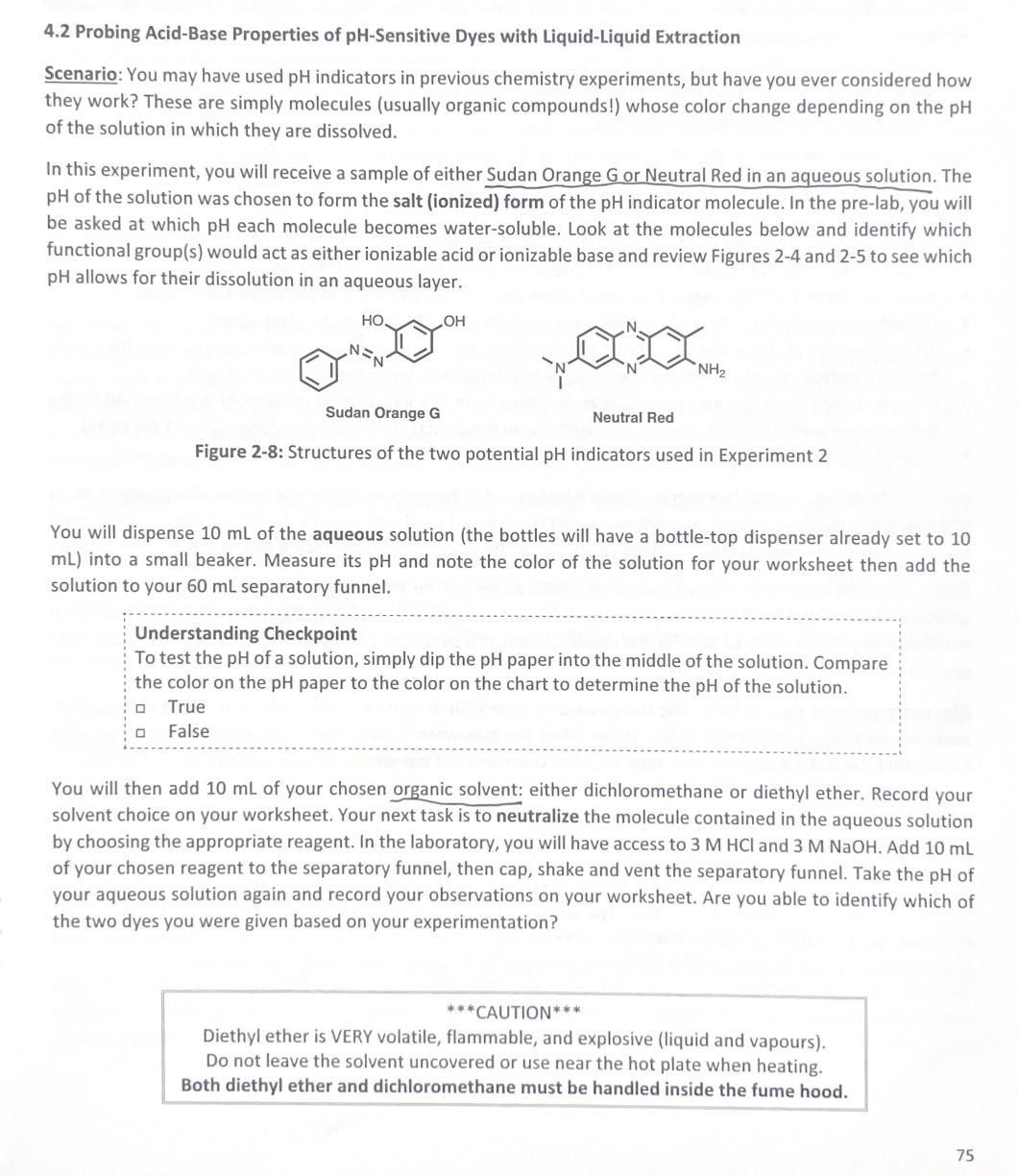
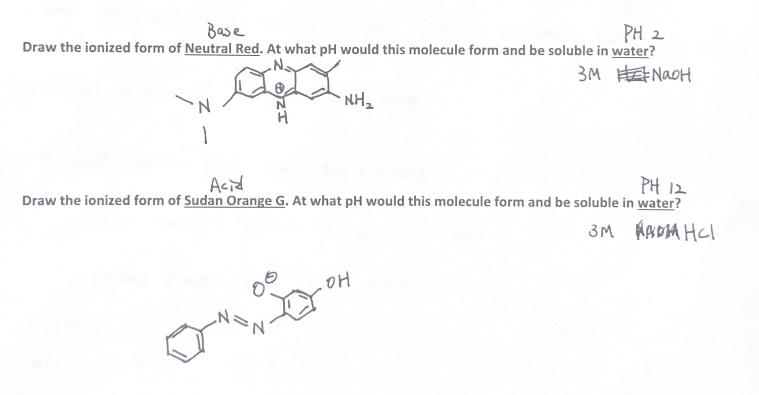
Question: 4.2 Probing Acid-Base Properties of pH-Sensitive Dyes with Liquid-Liquid Extraction Scen ario: You may have used pH indicators in previous chemistry experiments, but have you

Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


