Question: answer all for rating The figure below is a typical titration of a weak acid and a strong base a. The endpoint is identified as
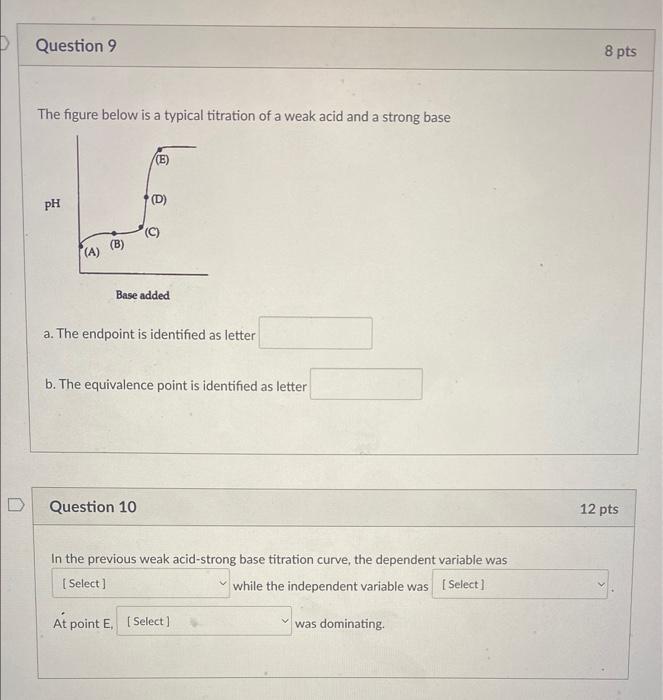
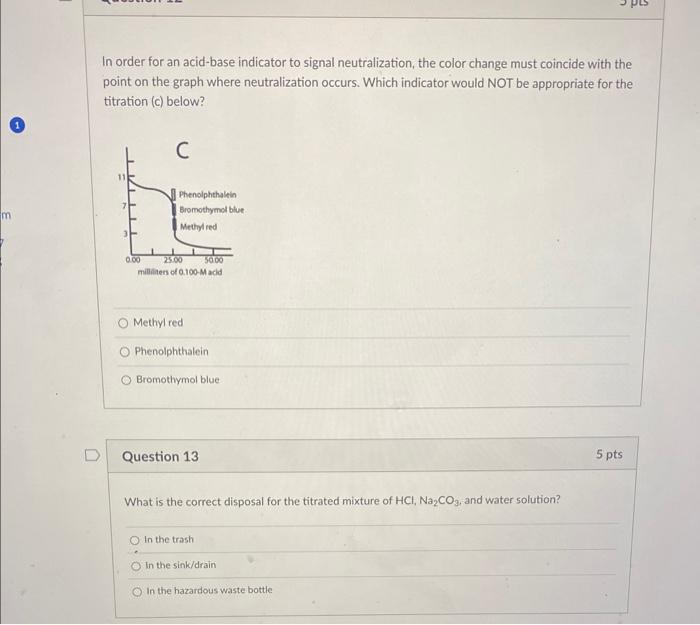
The figure below is a typical titration of a weak acid and a strong base a. The endpoint is identified as letter b. The equivalence point is identified as letter Question 10 12 pts In the previous weak acid-strong base titration curve, the dependent variable was while the independent variable was At point E was dominating. In order for an acid-base indicator to signal neutralization, the color change must coincide with the point on the graph where neutralization occurs. Which indicator would NOT be appropriate for the titration (c) below? Methyl red Phenolphthalein Bromothymol blue Question 13 5pts What is the correct disposal for the titrated mixture of HCl,Na2CO3. and water solution? In the trash In the sink/drain In the hazardous waste bottle
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


