Question: can someone answer these so i am able to compare answers? weak acid - strong base - equivalence point - 2. Write a molecular, an
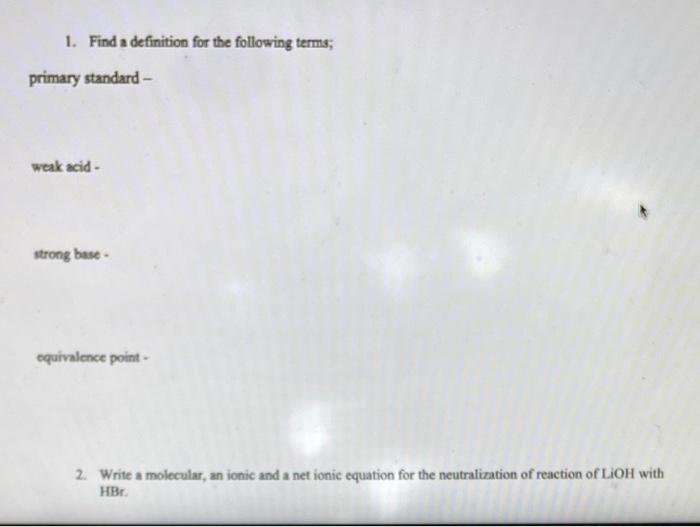
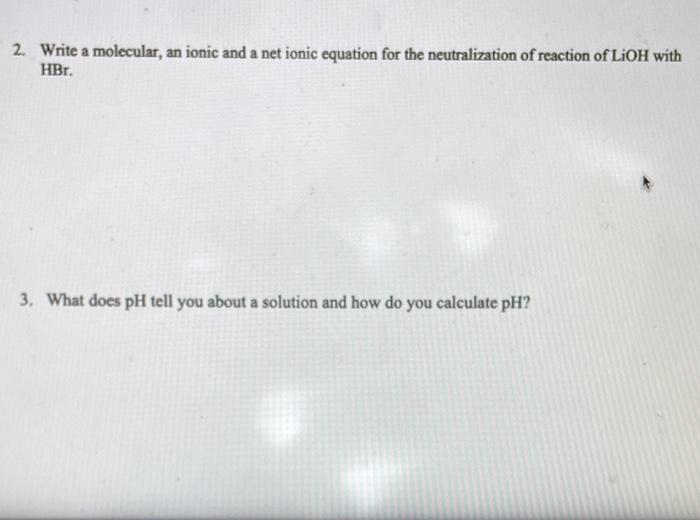


weak acid - strong base - equivalence point - 2. Write a molecular, an ionic and a net ionic equation for the neutralization of reaction of LiOH with HBr. 2. Write a molecular, an ionic and a net ionic equation for the neutralization of reaction of LiOH with HBr. 3. What does pH tell you about a solution and how do you calculate pH ? 4. Titration. A 5.00mL sample of hydrochloric acid, HCl was neutralized by titration of 34.25mL of 0.105MNaOH. a) Write the balanced equation of this reaction. b) Calculate how many moles of NaOH were used in this reaction. c) How many moles of HCl were in a sample? d) What is a concentration of HCl in a 5.00mL sample? 5. Calculate the mass of potassium hydrogen phthalate (KHP), KHC1H2O4 needed to react completely with 20.00mL of 0.10MNaOH solution. The equation is given in the lab manual. (MM of KHP is 204.2g/mol )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


