Question: Answer all of this please. 1- For an aqueous solution of sucrose (C12H22O11), determine (a) the molarity of 5.00L of a solution that contains 235g
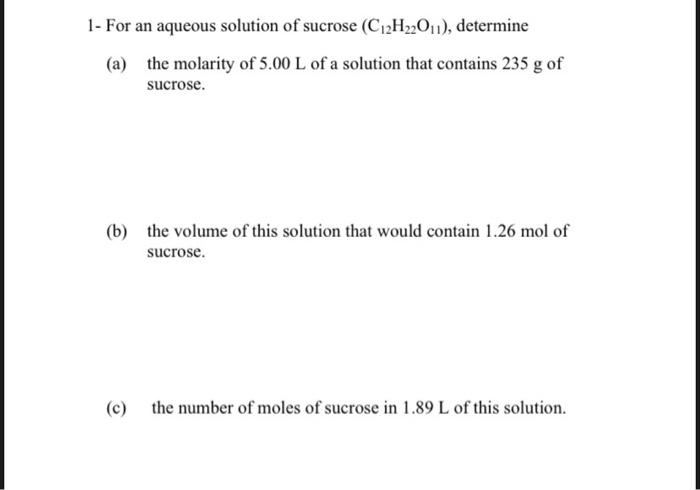
1- For an aqueous solution of sucrose (C12H22O11), determine (a) the molarity of 5.00L of a solution that contains 235g of sucrose. (b) the volume of this solution that would contain 1.26mol of sucrose. (c) the number of moles of sucrose in 1.89L of this solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


