Question: answer all please Give the ground-state electron configuration for each of the following elements. After each atom is its atomic number in parentheses. (a) Potassium


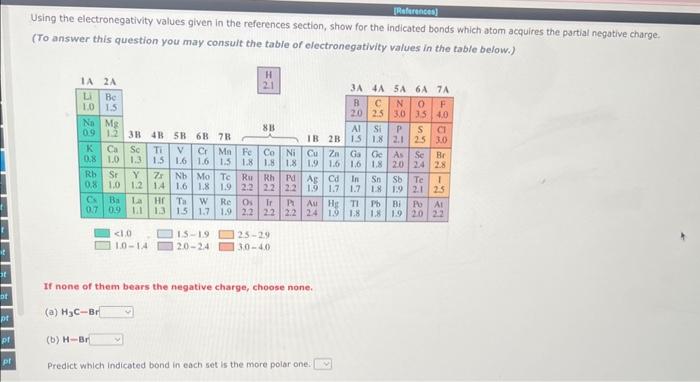
Give the ground-state electron configuration for each of the following elements. After each atom is its atomic number in parentheses. (a) Potassium (19) (b) Carbon (6) 9 item attempts remaining Specify the number of unshared pairs of electrons necessary to complete the valence shiell of the labeled atoms, a-c in the foliowing structures. 5 pecily "o if no palr of electron needs to be added. Notice that formal charges are indicated by or and that the atoms are NOT labeled in sequence. 1. a b 2. b c Using the electronegativity values given in the references section, show for the indicated bonds which atom acquires the partial negative charge. (To answer this question you may consult the table of electronegativity values in the table below.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


