Question: ANSWER ALL!!! QUESTION 1 For the reaction A+B -> C, the rate law is given below. Determine the rate of the reaction when the concentration
ANSWER ALL!!!
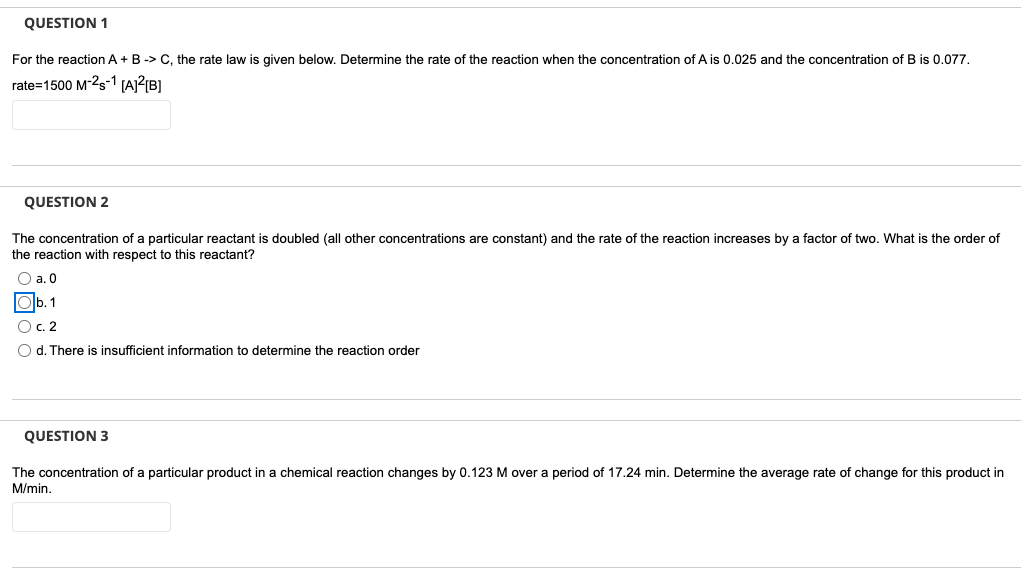

QUESTION 1 For the reaction A+B -> C, the rate law is given below. Determine the rate of the reaction when the concentration of Ais 0.025 and the concentration of B is 0.077. rate=1500 M-25-1 [A]2[B] QUESTION 2 The concentration of a particular reactant is doubled (all other concentrations are constant) and the rate of the reaction increases by a factor of two. What is the order of the reaction with respect to this reactant? O a. O 1 O c. 2 O d. There is insufficient information to determine the reaction order QUESTION 3 The concentration of a particular product in a chemical reaction changes by 0.123 M over a period of 17.24 min. Determine the average rate of change for this product in M/min. QUESTION 4 For which reaction order(s) is the half-life independent of the initial concentration? a. Zero O b. 2nd O c. 1st O d. All of the above QUESTION 5 Which of the equations below would be appropriate to use for a second order reaction. Oa. [1] In -=-kt [1] Ob. 1 1 [A] [A] c. In[A], = - kt+In[A], O d. [A],= - kt+[A] =kt +
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


