Question: answer all three and show work please How many solution is needed to completely neutralize mL of a solution according to the balanced chemical reaction:
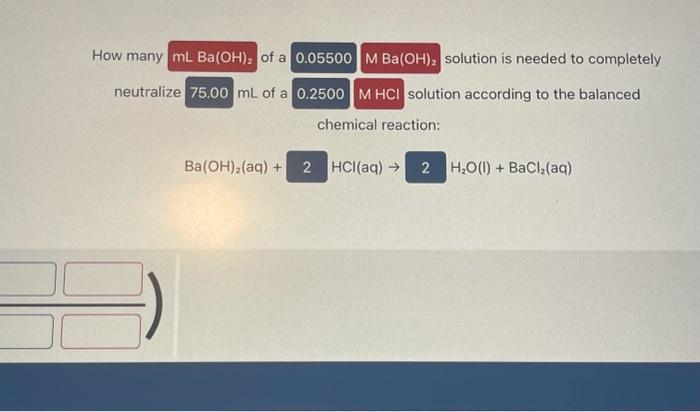
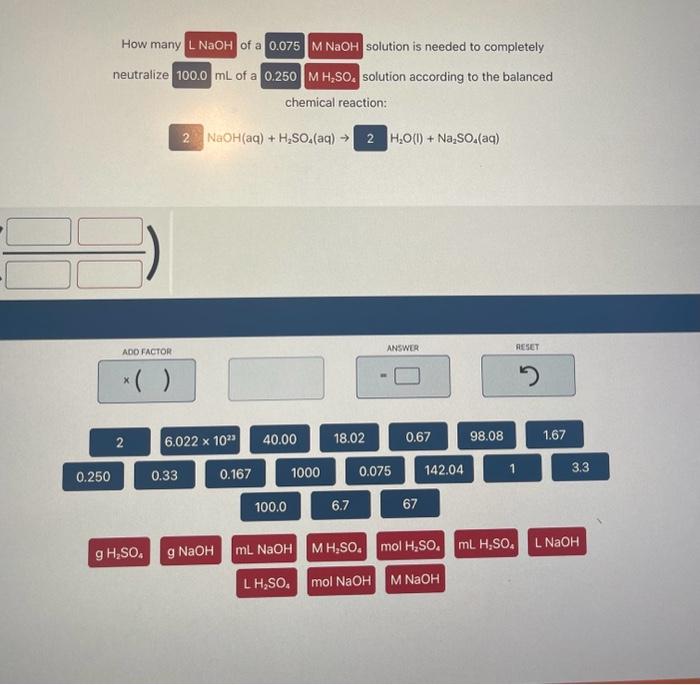
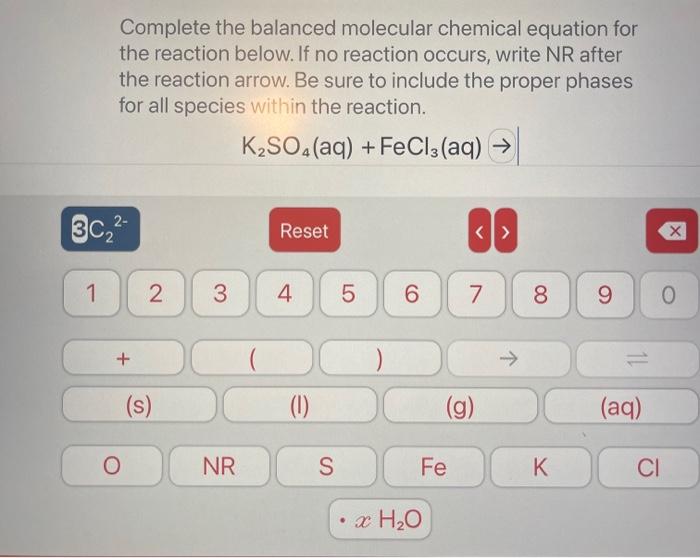
How many solution is needed to completely neutralize mL of a solution according to the balanced chemical reaction: Ba(OH)2(aq)+HCl(aq)H2O(I)+BaCl2(aq) How many of a 0.075MNaOH solution is needed to completely neutralize mL of a 0.250MH2SO4 solution according to the balanced chemical reaction: NaOH(aq)+H2SO4(aq)H2O(l)+Na2SO4(aq) Complete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. Be sure to include the proper phases for all species within the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


