Question: answer e to ii please 21 1. A student performs a redox titration between 10.0 mL of hypochlorous acid, HCIO(aq), and chromium(III) nitrate, Cr(NO3)3(aq). The
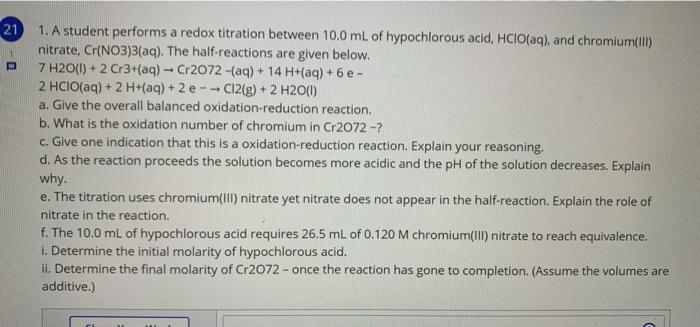
21 1. A student performs a redox titration between 10.0 mL of hypochlorous acid, HCIO(aq), and chromium(III) nitrate, Cr(NO3)3(aq). The half-reactions are given below. 7 H20(1) + 2 Cr3+(aq) -- Cr2072-(aq) + 14 H+(aq) + 6 e- 2 HCIO(aq) + 2 H+(aq) + 2 e-- C12(g) + 2 H20(1) a. Give the overall balanced oxidation-reduction reaction. b. What is the oxidation number of chromium in Cr2072 -? c. Give one indication that this is a oxidation-reduction reaction. Explain your reasoning. d. As the reaction proceeds the solution becomes more acidic and the pH of the solution decreases. Explain why. e. The titration uses chromium(Ill) nitrate yet nitrate does not appear in the half-reaction. Explain the role of nitrate in the reaction. f. The 100 mL of hypochlorous acid requires 26.5 mL of 0.120 M chromium(III) nitrate to reach equivalence. 1. Determine the initial molarity of hypochlorous acid. il. Determine the final molarity of Cr2O72- once the reaction has gone to completion. (Assume the volumes are additive.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


