Question: Answer Each Question About The Molecule 1-Buten-3-Yne (Shown Below) By Typing A Number Or A Hybrid Orbital Designation (Sp, Sp2, Sp3, Sp3d, Or Sp3d2) Into
Answer Each Question About The Molecule 1-Buten-3-Yne (Shown Below) By Typing A Number Or A Hybrid Orbital Designation (Sp, Sp2, Sp3, Sp3d, Or Sp3d2) Into The Appropriate Space. LOOK AT THIS BOND!!! H H-Ec- CH H The Total Number Of Sigma Bonds Present In This Molecule Is: The Total Number Of Pi Bonds Present In This Molecule Is: In The Bond Being Pointed To
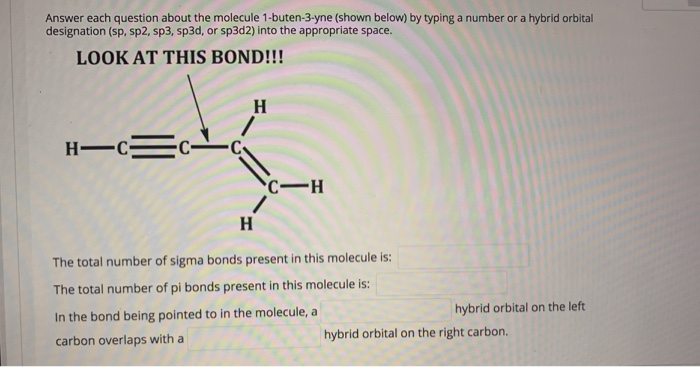
Answer each question about the molecule 1-buten-3-yne (shown below) by typing a number or a hybrid orbital designation (sp, sp2, sp3, sp3d, or sp3d2) into the appropriate space. LOOK AT THIS BOND!!! H H-C=C- C-H H The total number of sigma bonds present in this molecule is: The total number of pi bonds present in this molecule is: In the bond being pointed to in the molecule, a carbon overlaps with a hybrid orbital on the left hybrid orbital on the right carbon.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


